Page 135 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 135
98 Carraher’s Polymer Chemistry
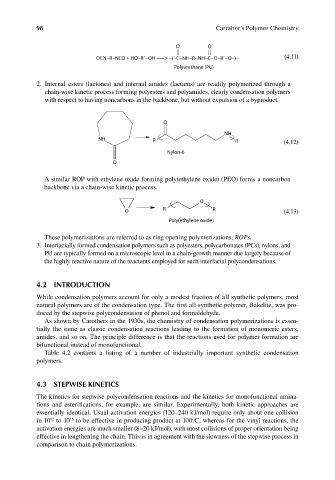
O O
OCN−R−NCO + HO−R −OH −> −(−C−NH−R−NH−C−O−R −O−)− (4.11)
Polyurethane (PU)
2. Internal esters (lactones) and internal amides (lactams) are readily polymerized through a
chain-wise kinetic process forming polyesters and polyamides, clearly condensation polymers
with respect to having noncarbons in the backbone, but without expulsion of a byproduct.
O
NH
NH R R (4.12)
Nylon-6
O
A similar ROP with ethylene oxide forming poly(ethylene oxide) (PEO) forms a noncarbon
backbone via a chain-wise kinetic process.
O
R R
O (4.13)
Poly(ethylene oxide)
These polymerizations are referred to as ring opening polymerizations, ROPs.
3. Interfacially formed condensation polymers such as polyesters, polycarbonates (PCs), nylons, and
PU are typically formed on a microscopic level in a chain-growth manner due largely because of
the highly reactive nature of the reactants employed for such interfacial polycondensations.
4.2 INTRODUCTION
While condensation polymers account for only a modest fraction of all synthetic polymers, most
natural polymers are of the condensation type. The first all-synthetic polymer, Bakelite, was pro-
duced by the stepwise polycondensation of phenol and formaldehyde.
As shown by Carothers in the 1930s, the chemistry of condensation polymerizations is essen-
tially the same as classic condensation reactions leading to the formation of monomeric esters,
amides, and so on. The principle difference is that the reactions used for polymer formation are
bifunctional instead of monofunctional.
Table 4.2 contains a listing of a number of industrially important synthetic condensation
polymers.
4.3 STEPWISE KINETICS
The kinetics for stepwise polycondensation reactions and the kinetics for monofunctional amina-
tions and esterifications, for example, are similar. Experimentally, both kinetic approaches are
essentially identical. Usual activation energies (120–240 kJ/mol) require only about one collision
o
15
12
in 10 to 10 to be effective in producing product at 100 C, whereas for the vinyl reactions, the
activation energies are much smaller (8–20 kJ/mol), with most collisions of proper orientation being
effective in lengthening the chain. This is in agreement with the slowness of the stepwise process in
comparison to chain polymerizations.
9/14/2010 3:38:06 PM
K10478.indb 98 9/14/2010 3:38:06 PM
K10478.indb 98