Page 137 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 137
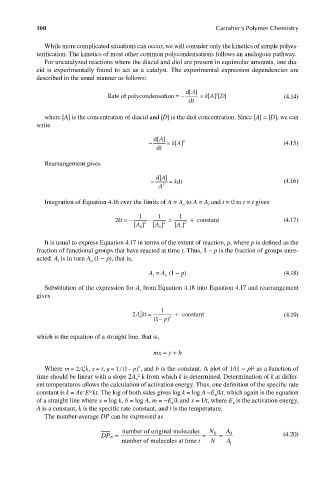
100 Carraher’s Polymer Chemistry
While more complicated situations can occur, we will consider only the kinetics of simple polyes-
terification. The kinetics of most other common polycondensations follows an analogous pathway.
For uncatalyzed reactions where the diacid and diol are present in equimolar amounts, one dia-
cid is experimentally found to act as a catalyst. The experimental expression dependencies are
described in the usual manner as follows:
A
Rate of polycondensation =− d[ ] = [ ] [ ] (4.14)
D
kA
2
dt
where [A] is the concentration of diacid and [D] is the diol concentration. Since [A] = [D], we can
write
A
− d[ ] = kA 3 (4.15)
[]
dt
Rearrangement gives
A
− d[ ] = kt (4.16)
d
A 3
Integration of Equation 4.16 over the limits of A = A to A = A and t = 0 to t = t gives
o t
2kt =− 1 − 1 = 1 + constant (4.17)
A
[A ] 2 [A ] 2 [ ] 2
0 0 t
It is usual to express Equation 4.17 in terms of the extent of reaction, p, where p is defi ned as the
fraction of functional groups that have reacted at time t. Thus, 1 − p is the fraction of groups unre-
acted. A is in turn A (1 − p), that is,
t o
A = A (1 − p) (4.18)
t o
Substitution of the expression for A from Equation 4.18 into Equation 4.17 and rearrangement
t
gives
2Akt = 1 + constant (4.19)
2
−
0 (1 p ) 2
which is the equation of a straight line, that is,
mx = y + b
−
Where m = 2A k x = , t y = 1/(1 p ) , and b is the constant. A plot of 1/(1 − p) as a function of
2
2
2
,
0
2
time should be linear with a slope 2A k from which k is determined. Determination of k at differ-
o
ent temperatures allows the calculation of activation energy. Thus, one definition of the specifi c rate
a/
–
constant is k = Ae E kt. The log of both sides gives log k = log A –E /kt, which again is the equation
a
of a straight line where y = log k, b = log A, m = −E /k and x = 1/t, where E is the activation energy,
a a
A is a constant, k is the specific rate constant, and t is the temperature.
The number-average DP can be expressed as
DP n = number of original molecules = N 0 = A 0 (4.20)
number of molecules at time t N A t
9/14/2010 3:38:07 PM
K10478.indb 100 9/14/2010 3:38:07 PM
K10478.indb 100