Page 142 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 142
Polycondensation Polymers 105
4.5 POLYESTERS
Carothers and his research group at DuPont began to investigate the formation of polymers
from the reaction of aliphatic diacids with diols, generally adipic acid and ethylene glycol
(derived from reaction of ethylene oxide with water; major ingredient in most antifreeze), in
search of materials that would give them fibers. They were only able to form syrupy mixtures.
This is because, unlike reactions with diamines (Section 4.7), the equilibrium reaction greatly
disfavors ester formation. Further, the ability to have almost equal amounts of functional groups
is easily achieved with the amines through formation of salts as shown in Equation 4.53, but diols
do not form such salts. The critical need to have the reactants present in equal molar amounts
for equilibrium determined reactions is clearly seen in Equation 4.21. Carothers’ group under-
stood the principle of “driving” an equilibrium reaction so sought to remove water thus forcing
the reaction toward ester formation. For this they developed a so-called “molecular still” which
was simply heating the mixture and applying a vacuum coupled with a “cold-finger” that allowed
evacuated water to condense and be removed from the reaction system. Since the fractional con-
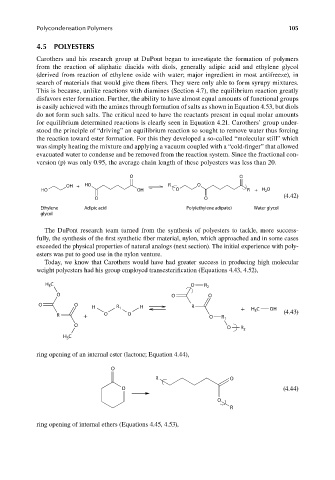
version (p) was only 0.95, the average chain length of these polyesters was less than 20.
O O
OH + HO R O
HO OH O R + H 2 O
(4.42)
O O
Ethylene Adipic acid Poly(ethylene adipate) Water glycol
glycol
The DuPont research team turned from the synthesis of polyesters to tackle, more success-
fully, the synthesis of the first synthetic fiber material, nylon, which approached and in some cases
exceeded the physical properties of natural analogs (next section). The initial experience with poly-
esters was put to good use in the nylon venture.
Today, we know that Carothers would have had greater success in producing high molecular
weight polyesters had his group employed transesterification (Equations 4.43, 4.52),
H 3 C O R 2
O O O
O O
H R 1 H R + H 3 C OH
R + O O O R 1 (4.43)
O O R 2
H 3 C
ring opening of an internal ester (lactone; Equation 4.44),
O
R O
O (4.44)
O
R
ring opening of internal ethers (Equations 4.45, 4.53),
9/14/2010 3:38:18 PM
K10478.indb 105 9/14/2010 3:38:18 PM
K10478.indb 105