Page 145 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 145
108 Carraher’s Polymer Chemistry
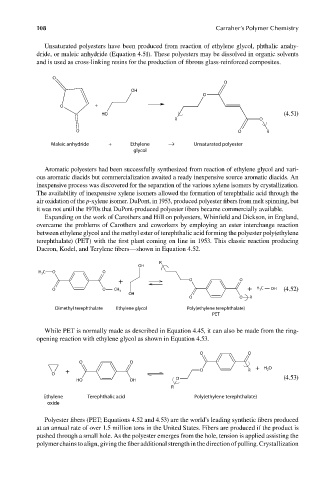
Unsaturated polyesters have been produced from reaction of ethylene glycol, phthalic anahy-
dride, or maleic anhydride (Equation 4.51). These polyesters may be dissolved in organic solvents
and is used as cross-linking resins for the production of fibrous glass-reinforced composites.
O
O
OH
O
O +
HO (4.51)
R O
O O R
Maleic anhydride + Ethylene → Unsaturated polyester
glycol
Aromatic polyesters had been successfully synthesized from reaction of ethylene glycol and vari-
ous aromatic diacids but commercialization awaited a ready inexpensive source aromatic diacids. An
inexpensive process was discovered for the separation of the various xylene isomers by crystallization.
The availability of inexpensive xylene isomers allowed the formation of terephthalic acid through the
air oxidation of the p-xylene isomer. DuPont, in 1953, produced polyester fibers from melt spinning, but
it was not until the 1970s that DuPont-produced polyester fibers became commercially available.
Expanding on the work of Carothers and Hill on polyesters, Whinfield and Dickson, in England,
overcame the problems of Carothers and coworkers by employing an ester interchange reaction
between ethylene glycol and the methyl ester of terephthalic acid forming the polyester poly(ethylene
terephthalate) (PET) with the first plant coming on line in 1953. This classic reaction producing
Dacron, Kodel, and Terylene fibers—shown in Equation 4.52.
R
OH
H 3 C O O
+ O O
O O CH 3 + H 3 C OH (4.52)
OH
O O R
Dimethyl terephthalate Ethylene glycol Poly(ethylene terephthalate)
PET
While PET is normally made as described in Equation 4.45, it can also be made from the ring-
opening reaction with ethylene glycol as shown in Equation 4.53.
O O
O O
+ O R + H O
2
O
HO OH O (4.53)
R
Ethylene Terephthalic acid Poly(ethylene terephthalate)
oxide
Polyester fibers (PET; Equations 4.52 and 4.53) are the world’s leading synthetic fi bers produced
at an annual rate of over 1.5 million tons in the United States. Fibers are produced if the product is
pushed through a small hole. As the polyester emerges from the hole, tension is applied assisting the
polymer chains to align, giving the fiber additional strength in the direction of pulling. Crystallization
9/14/2010 3:38:19 PM
K10478.indb 108 9/14/2010 3:38:19 PM
K10478.indb 108