Page 144 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 144
Polycondensation Polymers 107
−
H O
(4.49)
R H
HO O
Reaction with anhydrides and acid chlorides are more rapid and can occur in an essentially
nonreversible fashion. But, anhydrides and acid chlorides are considered so-called “high energy”
reactants since they often involve additional energy-requiring steps in their production and thus are
less suitable for large-scale production of materials. The activity energies for direct esterifi cation
and transesterification are on the order of 30 kcal/mole (120 kJ/mole) while the activation energies
for anhydride and acid chloride reaction with alcohols are on the order of 15–20 kcal/mole (60–80
kJ/mole).
The initial polyester formation actually occurred early and is attributed to Gay Lussac and
Pelouze in 1833 and Berzelius in 1847. These polyesters are called glyptals and alkyds, and they
are useful as coatings materials and not for fiber production. While these reactions had low frac-
tional conversions, they formed high molecular weight materials because they had funtionalities
(i.e., number of reactive groups on a single reactant) greater than two resulting in cross-linking.
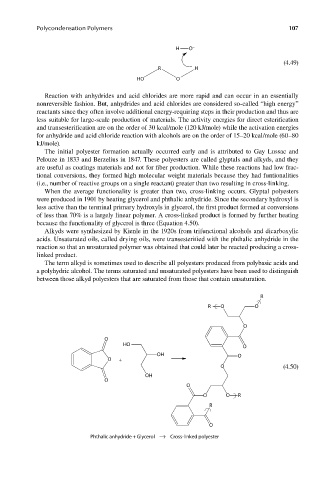
When the average functionality is greater than two, cross-linking occurs. Glyptal polyesters
were produced in 1901 by heating glycerol and phthalic anhydride. Since the secondary hydroxyl is
less active than the terminal primary hydroxyls in glycerol, the first product formed at conversions
of less than 70% is a largely linear polymer. A cross-linked product is formed by further heating
because the functionality of glycerol is three (Equation 4.50).
Alkyds were synthesized by Kienle in the 1920s from trifunctional alcohols and dicarboxylic
acids. Unsaturated oils, called drying oils, were transesterified with the phthalic anhydride in the
reaction so that an unsaturated polymer was obtained that could later be reacted producing a cross-
linked product.
The term alkyd is sometimes used to describe all polyesters produced from polybasic acids and
a polyhydric alcohol. The terms saturated and unsaturated polyesters have been used to distinguish
between those alkyd polyesters that are saturated from those that contain unsaturation.
R
R O O
O
O
HO
O
OH O
O +
O (4.50)
OH
O
O
O O R
R
O
Phthalic anhydride + Glycerol → Cross-linked polyester
9/14/2010 3:38:19 PM
K10478.indb 107 9/14/2010 3:38:19 PM
K10478.indb 107