Page 141 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 141
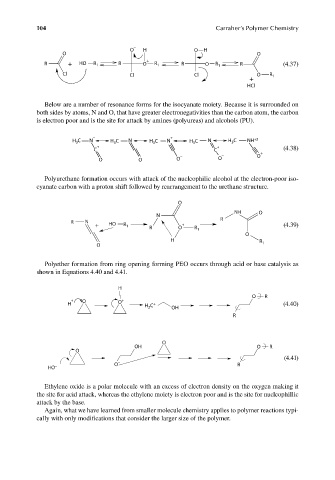
104 Carraher’s Polymer Chemistry
−
O H O H
O O
R + HO R 1 R O + R 1 R O R 1 R (4.37)
Cl Cl Cl O R 1
+
HCl
Below are a number of resonance forms for the isocyanate moiety. Because it is surrounded on
both sides by atoms, N and O, that have greater electronegativities than the carbon atom, the carbon
is electron poor and is the site for attack by amines (polyureas) and alcohols (PU).
− +
C N N N N H C NH +2
H 3 H C H 3 C H C 3
3
3
+ +
C C (4.38)
− − O +
O O O O
Polyurethane formation occurs with attack of the nucleophilic alcohol at the electron-poor iso-
cyanate carbon with a proton shift followed by rearrangement to the urethane structure.
O
− NH O
N
R N R
+ HO R + (4.39)
1
R O R 1
O
H R
O 1
Polyether formation from ring opening forming PEO occurs through acid or base catalysis as
shown in Equations 4.40 and 4.41.
H
O R
+ O +
H O H C + OH (4.40)
2
R
O
OH O R
O
(4.41)
−
− O R
HO
Ethylene oxide is a polar molecule with an excess of electron density on the oxygen making it
the site for acid attack, whereas the ethylene moiety is electron poor and is the site for nucleophillic
attack by the base.
Again, what we have learned from smaller molecule chemistry applies to polymer reactions typi-
cally with only modifications that consider the larger size of the polymer.
9/14/2010 3:38:17 PM
K10478.indb 104
K10478.indb 104 9/14/2010 3:38:17 PM