Page 139 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 139
102 Carraher’s Polymer Chemistry
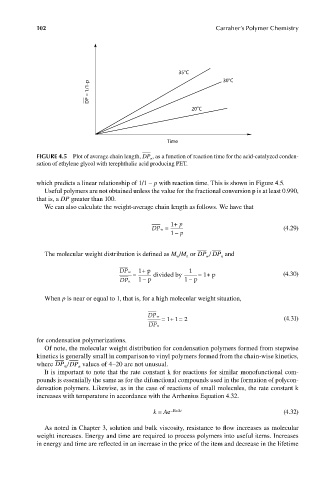
35 C 30 C
DP = 1/1-p
20 C
Time
FIGURE 4.5 Plot of average chain length, DP , as a function of reaction time for the acid-catalyzed conden-
n
sation of ethylene glycol with terephthalic acid producing PET.
which predicts a linear relationship of 1/1 − p with reaction time. This is shown in Figure 4.5.
Useful polymers are not obtained unless the value for the fractional conversion p is at least 0.990,
that is, a DP greater than 100.
We can also calculate the weight-average chain length as follows. We have that
+
1 p
DP w = 1 − (4.29)
p
The molecular weight distribution is defi ned as M /M or DP / DP and
n
n
w
w
+
DP w 1p 1
= divided by = + (4.30)
1 p
DP n 1 − p 1 − p
When p is near or equal to 1, that is, for a high molecular weight situation,
DP
w
=+= 2 (4.31)
11
DP n
for condensation polymerizations.
Of note, the molecular weight distribution for condensation polymers formed from stepwise
kinetics is generally small in comparison to vinyl polymers formed from the chain-wise kinetics,
where DP /DP values of 4–20 are not unusual.
w
n
It is important to note that the rate constant k for reactions for similar monofunctional com-
pounds is essentially the same as for the difunctional compounds used in the formation of polycon-
densation polymers. Likewise, as in the case of reactions of small molecules, the rate constant k
increases with temperature in accordance with the Arrhenius Equation 4.32.
k = Ae –Ea/kt (4.32)
As noted in Chapter 3, solution and bulk viscosity, resistance to flow increases as molecular
weight increases. Energy and time are required to process polymers into useful items. Increases
in energy and time are reflected in an increase in the price of the item and decrease in the lifetime
9/14/2010 3:38:13 PM
K10478.indb 102 9/14/2010 3:38:13 PM
K10478.indb 102