Page 140 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 140
Polycondensation Polymers 103
of the equipment used to process the polymers. There is need to produce polymeric materials that
exhibit the needed physical properties and yet have a reasonable viscosity to minimize the energy
and time required to produce the particular product. Thus, molecular weight control is important.
We have already looked the major indicator of molecular weight control. It is the Carothers’ rela-
tionship DP = 1/1 − p so that chain length can be controlled through extent of reaction and purity
n
of reactants. This relationship was initially one of the least understood relationships and, early in
the commercial production of polymers, costed industry a lot of time and money because of a lack
of understanding its importance. Its importance is now well understood. Thus, the extent of reaction
can be controlled through simply stopping the reaction by cooling it. More typically, it is controlled
through the use of an excess of one or the other reactant or the introduction of some monofunctional
reactant that will halt chain growth when it is added to a growing polymer chain.
For the situation when an excess of one reactant is employed, chain growth continues until all
of the reactant that is present in the smaller molar amount reacts leaving chains with end groups
derived from the reactant that is present in excess. For a reaction where the number of moles of
“B” are in excess, N > N , the extent of reaction for A is p and for B it is “rp” where r is the ratio
B A
of N /N The following relationship can be derived.
A B.
1 r
+
DP n = 1 r+− (4.33)
2rp
For an excess in N of one mole percent we have that r = 100/101 = 0.9901 and
B
+
DP n = 1 0.9901 = 201 (4.34)
−
+
1 0.9901 2(0.9901)(1)
4.4 POLYCONDENSATION MECHANISMS
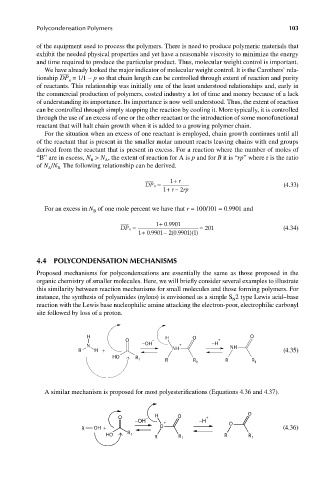
Proposed mechanisms for polycondensations are essentially the same as those proposed in the
organic chemistry of smaller molecules. Here, we will briefly consider several examples to illustrate
this similarity between reaction mechanisms for small molecules and those forming polymers. For
instance, the synthesis of polyamides (nylons) is envisioned as a simple S 2 type Lewis acid–base
N
reaction with the Lewis base nucleophilic amine attacking the electron-poor, electrophilic carbonyl
site followed by loss of a proton.
H H O O
O − +
N −OH + −H NH
R H + NH (4.35)
HO R 1 R R 1 R R 1
A similar mechanism is proposed for most polyesterifications (Equations 4.36 and 4.37).
O H O O
+ O
R OH + O (4.36)
R
HO 1 R R
R R 1 1
9/14/2010 3:38:16 PM
K10478.indb 103
K10478.indb 103 9/14/2010 3:38:16 PM