Page 143 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 143
106 Carraher’s Polymer Chemistry
O R
OH
O O
O O O
+ R 1 (4.45)
R 1
O
OH
R
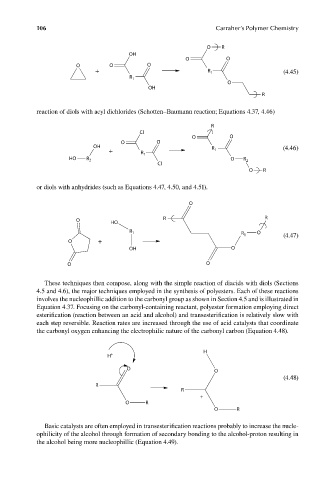
reaction of diols with acyl dichlorides (Schotten–Baumann reaction; Equations 4.37, 4.46)
R
Cl
O O
O O
OH R (4.46)
+ R 1
HO R 2 1 O R 2
Cl
O R
or diols with anhydrides (such as Equations 4.47, 4.50, and 4.51).
O
O R R
HO
R 1 R 1 O (4.47)
O +
OH O
O O
These techniques then compose, along with the simple reaction of diacids with diols (Sections
4.5 and 4.6), the major techniques employed in the synthesis of polyesters. Each of these reactions
involves the nucleophillic addition to the carbonyl group as shown in Section 4.5 and is illustrated in
Equation 4.37. Focusing on the carbonyl-containing reactant, polyester formation employing direct
esterification (reaction between an acid and alcohol) and transesterification is relatively slow with
each step reversible. Reaction rates are increased through the use of acid catalysts that coordinate
the carbonyl oxygen enhancing the electrophilic nature of the carbonyl carbon (Equation 4.48).
+ H
H
O O
(4.48)
R
R
+
O R
O R
Basic catalysts are often employed in transesterification reactions probably to increase the nucle-
ophilicity of the alcohol through formation of secondary bonding to the alcohol-proton resulting in
the alcohol being more nucleophillic (Equation 4.49).
9/14/2010 3:38:18 PM
K10478.indb 106 9/14/2010 3:38:18 PM
K10478.indb 106