Page 133 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 133
96 Carraher’s Polymer Chemistry
R R R R R R
R R R R R
R R R R R R R R
R R R R R R
R R R R R R
R R R R
R R R R R R R R R R R R
R R R R R R R R R
R R R
R R R
R R R R R R
R R R R R R R
R R R
R R R R R R R
R R R R R R R R R
R R R R
R R R R R R R R R R R
R R R R
R R R R R R R
R R R R R R R
R R R R R R R
R R R R R R R
R R
R R R
R R R R R R R
R R R R R R R
R R R R
R R R R R R R R R
R R R R R
R R R
R R R R R R R R R R R
R R R R
R R R R R R R R R
R R R R R R R R R R R R R R
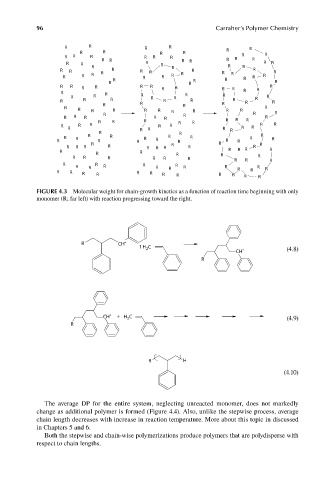
FIGURE 4.3 Molecular weight for chain-growth kinetics as a function of reaction time beginning with only
monomer (R; far left) with reaction progressing toward the right.
R CH •
1 H C • (4.8)
2
CH
R
•
CH + H 2 C (4.9)
R
R H
(4.10)
The average DP for the entire system, neglecting unreacted monomer, does not markedly
change as additional polymer is formed (Figure 4.4). Also, unlike the stepwise process, average
chain length decreases with increase in reaction temperature. More about this topic in discussed
in Chapters 5 and 6.
Both the stepwise and chain-wise polymerizations produce polymers that are polydisperse with
respect to chain lengths.
9/14/2010 3:38:05 PM
K10478.indb 96
K10478.indb 96 9/14/2010 3:38:05 PM