Page 202 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 202
Ionic Chain-Reaction and Complex Coordination Polymerization 165
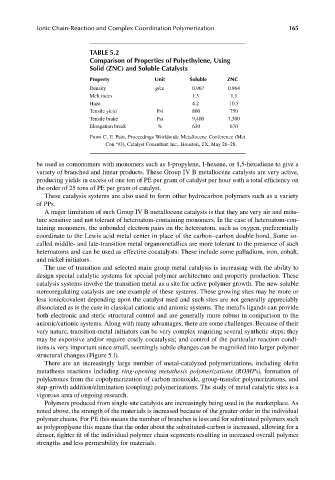
TABLE 5.2
Comparison of Properties of Polyethylene, Using
Solid (ZNC) and Soluble Catalysts
Property Unit Soluble ZNC
Density g/cc 0.967 0.964
Melt index 1.3 1.1
Haze 4.2 10.5
Tensile yield Psi 800 750
Tensile brake Psi 9,400 7,300
Elongation break % 630 670
From C. F. Pain, Proceedings Worldwide Metallocene Conference (Met
Con ‘93), Catalyst Consultant Inc., Houston, TX, May 26–28.
be used as comonomers with monomers such as 1-propylene, 1-hexene, or 1,5-hexadiene to give a
variety of branched and linear products. These Group IV B metallocene catalysts are very active,
producing yields in excess of one ton of PE per gram of catalyst per hour with a total effi ciency on
the order of 25 tons of PE per gram of catalyst.
These catalysis systems are also used to form other hydrocarbon polymers such as a variety
of PPs.
A major limitation of such Group IV B metallocene catalysts is that they are very air and mois-
ture sensitive and not tolerant of heteroatom-containing monomers. In the case of heteroatom-con-
taining monomers, the unbonded electron pairs on the heteroatom, such as oxygen, preferentially
coordinate to the Lewis acid metal center in place of the carbon–carbon double bond. Some so-
called middle- and late-transition metal organometallics are more tolerant to the presence of such
heteroatoms and can be used as effective cocatalysts. These include some palladium, iron, cobalt,
and nickel initiators.
The use of transition and selected main group metal catalysis is increasing with the ability to
design special catalytic systems for special polymer architecture and property production. These
catalysis systems involve the transition metal as a site for active polymer growth. The new soluble
stereoregulating catalysts are one example of these systems. These growing sites may be more or
less ionic/covalent depending upon the catalyst used and such sites are not generally appreciably
dissociated as is the case in classical cationic and anionic systems. The metal’s ligands can provide
both electronic and steric structural control and are generally more robust in comparison to the
anionic/cationic systems. Along with many advantages, there are some challenges. Because of their
very nature, transition-metal initiators can be very complex requiring several synthetic steps; they
may be expensive and/or require costly cocatalysts; and control of the particular reaction condi-
tions is very important since small, seemingly subtle changes can be magnified into larger polymer
structural changes (Figure 5.1).
There are an increasingly large number of metal-catalyzed polymerizations, including olefi n
metathesis reactions including ring-opening metathesis polymerizations (ROMPs), formation of
polyketones from the copolymerization of carbon monoxide, group-transfer polymerizations, and
step-growth addition/elimination (coupling) polymerizations. The study of metal catalytic sites is a
vigorous area of ongoing research.
Polymers produced from single-site catalysts are increasingly being used in the marketplace. As
noted above, the strength of the materials is increased because of the greater order in the individual
polymer chains. For PE this means the number of branches is less and for substituted polymers such
as polyproplyene this means that the order about the substituted-carbon is increased, allowing for a
denser, tighter fit of the individual polymer chain segments resulting in increased overall polymer
strengths and less permeability for materials.
9/14/2010 3:39:02 PM
K10478.indb 165 9/14/2010 3:39:02 PM
K10478.indb 165