Page 197 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 197
160 Carraher’s Polymer Chemistry
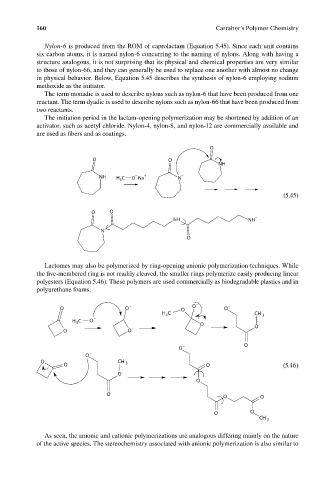
Nylon-6 is produced from the ROM of caprolactam (Equation 5.45). Since each unit contains
six carbon atoms, it is named nylon-6 concurring to the naming of nylons. Along with having a
structure analogous, it is not surprising that its physical and chemical properties are very similar
to those of nylon-66, and they can generally be used to replace one another with almost no change
in physical behavior. Below, Equation 5.45 describes the synthesis of nylon-6 employing sodium
methoxide as the initiator.
The term monadic is used to describe nylons such as nylon-6 that have been produced from one
reactant. The term dyadic is used to describe nylons such as nylon-66 that have been produced from
two reactants.
The initiation period in the lactam-opening polymerization may be shortened by addition of an
activator, such as acetyl chloride. Nylon-4, nylon-8, and nylon-12 are commercially available and
are used as fibers and as coatings.
O
O O
NH
−
NH H C O Na + N −
3
(5.45)
O O
NH NH −
N
O
Lactomes may also be polymerized by ring-opening anionic polymerization techniques. While
the five-membered ring is not readily cleaved, the smaller rings polymerize easily producing linear
polyesters (Equation 5.46). These polymers are used commercially as biodegradable plastics and in
polyurethane foams.
− O − −
O O O O
H C CH 3
3
−
H C O
3
O
O
O O
− O
O
−
O
O CH
O 3 O (5.46)
O
O
O O O
O O
CH 3
As seen, the anionic and cationic polymerizations are analogous differing mainly on the nature
of the active species. The stereochemistry associated with anionic polymerization is also similar to
9/14/2010 3:39:00 PM
K10478.indb 160 9/14/2010 3:39:00 PM
K10478.indb 160