Page 192 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 192
Ionic Chain-Reaction and Complex Coordination Polymerization 155
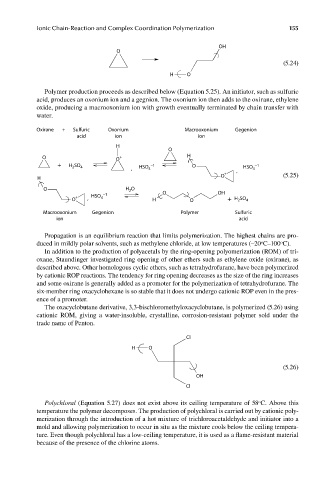
OH
O
(5.24)
H O
Polymer production proceeds as described below (Equation 5.25). An initiator, such as sulfuric
acid, produces an oxonium ion and a gegnion. The oxonium ion then adds to the oxirane, ethylene
oxide, producing a macrooxonium ion with growth eventually terminated by chain transfer with
water.
Oxirane + Sulfuric Oxonium Macrooxonium Gegenion
acid ion ion
H
O
O O + H
+ H 2 SO 4 HSO −1 O HSO −1
, 4 , 4
+
O (5.25)
H
O −1 H O O OH
2
O + , HSO 4 H O + H SO 4
2
Macrooxonium Gegenion Polymer Sulfuric
ion acid
Propagation is an equilibrium reaction that limits polymerization. The highest chains are pro-
o
o
duced in mildly polar solvents, such as methylene chloride, at low temperatures (−20 C–100 C).
In addition to the production of polyacetals by the ring-opening polyomerization (ROM) of tri-
oxane, Staundinger investigated ring opening of other ethers such as ethylene oxide (oxirane), as
described above. Other homologous cyclic ethers, such as tetrahydrofurane, have been polymerized
by cationic ROP reactions. The tendency for ring opening decreases as the size of the ring increases
and some oxirane is generally added as a promoter for the polymerization of tetrahydrofurane. The
six-member ring oxacyclohexane is so stable that it does not undergo cationic ROP even in the pres-
ence of a promoter.
The oxacyclobutane derivative, 3,3-bischloromethyloxacyclobutane, is polymerized (5.26) using
cationic ROM, giving a water-insoluble, crystalline, corrosion-resistant polymer sold under the
trade name of Penton.
Cl
H O
(5.26)
OH
Cl
o
Polychloral (Equation 5.27) does not exist above its ceiling temperature of 58 C. Above this
temperature the polymer decomposes. The production of polychloral is carried out by cationic poly-
merization through the introduction of a hot mixture of trichloroacetaldehyde and initiator into a
mold and allowing polymerization to occur in situ as the mixture cools below the ceiling tempera-
ture. Even though polychloral has a low-ceiling temperature, it is used as a fl ame-resistant material
because of the presence of the chlorine atoms.
9/14/2010 3:38:56 PM
K10478.indb 155
K10478.indb 155 9/14/2010 3:38:56 PM