Page 189 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 189
152 Carraher’s Polymer Chemistry
+
R = k [M][M ] (5.5)
p p
The termination rate R , assumed to be a first-order process, is simply the dissociation of the
t
macrocarbocation–gegenion complex here forming BF and H O and the now neutral “dead” poly-
3 2
mer chain. This is expressed as follows:
+
R = k [M ] (5.6)
t t
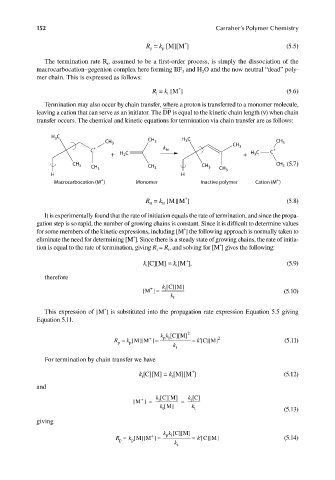
Termination may also occur by chain transfer, where a proton is transferred to a monomer molecule,
leaving a cation that can serve as an initiator. The DP is equal to the kinetic chain length (ν) when chain
transfer occurs. The chemical and kinetic equations for termination via chain transfer are as follows:
H C C
3
CH 3 CH 3 H 3 CH CH 3
C + k tr 3 C C +
+ H C + H 3
2
CH 3 CH 3 CH 3 CH 3 CH CH (5.7)
3
H H 3
+
+
Macrocarbocation (M ) Monomer Inactive polymer Cation (M )
+
R = k [M][M ] (5.8)
tr
tr
It is experimentally found that the rate of initiation equals the rate of termination, and since the propa-
gation step is so rapid, the number of growing chains is constant. Since it is difficult to determine values
+
for some members of the kinetic expressions, including [M ] the following approach is normally taken to
+
eliminate the need for determining [M ]. Since there is a steady state of growing chains, the rate of initia-
+
tion is equal to the rate of termination, giving R = R , and solving for [M ] gives the following:
t
i
+
k [C][M] = k [M ], (5.9)
i
t
therefore
+ k i [C][M]
[M ] = (5.10)
k t
+
This expression of [M ] is substituted into the propagation rate expression Equation 5.5 giving
Equation 5.11.
kk [C][M] 2
+ pi 2
k
R p = k p [M][M ] = = [C][M] (5.11)
k t
For termination by chain transfer we have
+
k [C][M] = k [M][M ] (5.12)
i
t
and
+ k i [C][M] k i [C]
[M ] = =
k [M] k
t t (5.13)
giving
+ kk [C][M]
pi
R p = k p [M][M ] = = k [C][M] (5.14)
k t
9/14/2010 3:38:52 PM
K10478.indb 152
K10478.indb 152 9/14/2010 3:38:52 PM