Page 188 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 188
Ionic Chain-Reaction and Complex Coordination Polymerization 151
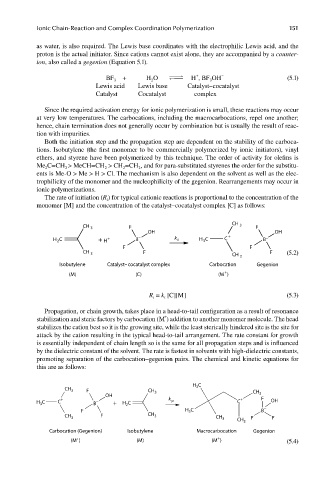
as water, is also required. The Lewis base coordinates with the electrophilic Lewis acid, and the
proton is the actual initiator. Since cations cannot exist alone, they are accompanied by a counter-
ion, also called a gegenion (Equation 5.1).
ZZX
–
+
BF + H O YZZ H , BF OH (5.1)
3 2 3
Lewis acid Lewis base Catalyst–cocatalyst
Catalyst Cocatalyst complex
Since the required activation energy for ionic polymerization is small, these reactions may occur
at very low temperatures. The carbocations, including the macrocarbocations, repel one another;
hence, chain termination does not generally occur by combination but is usually the result of reac-
tion with impurities.
Both the initiation step and the propagation step are dependent on the stability of the carboca-
tions. Isobutylene (the first monomer to be commercially polymerized by ionic initiators), vinyl
ethers, and styrene have been polymerized by this technique. The order of activity for olefi ns is
Me C=CH > MeCH=CH > CH =CH , and for para-substituted styrenes the order for the substitu-
2 2 2 2 2
ents is Me-O > Me > H > Cl. The mechanism is also dependent on the solvent as well as the elec-
trophilicity of the monomer and the nucleophilicity of the gegenion. Rearrangements may occur in
ionic polymerizations.
The rate of initiation (R ) for typical cationic reactions is proportional to the concentration of the
i
monomer [M] and the concentration of the catalyst–cocatalyst complex [C] as follows:
CH
CH 3 F 3 F
OH OH
– +
H C + H + B k i H C C B –
3
2
F F
CH 3 F CH 3 F (5.2)
Isobutylene Catalyst–cocatalyst complex Carbocation Gegenion
+
(M) (C) (M )
R = k [C][M] (5.3)
i
i
Propagation, or chain growth, takes place in a head-to-tail configuration as a result of resonance
+
stabilization and steric factors by carbocation (M ) addition to another monomer molecule. The head
stabilizes the cation best so it is the growing site, while the least sterically hindered site is the site for
attack by the cation resulting in the typical head-to-tail arrangement. The rate constant for growth
is essentially independent of chain length so is the same for all propagation steps and is infl uenced
by the dielectric constant of the solvent. The rate is fastest in solvents with high- dielectric constants,
promoting separation of the carbocation–gegenion pairs. The chemical and kinetic equations for
this are as follows:
H C
3
CH 3 F CH CH
OH 3 3
+ k + F
H C C B − + H C p C OH
3
2
F H 3 C B −
CH 3 F CH 3 F F
CH 3
CH 3
Carbocation (Gegenion) Isobutylene Macrocarbocation Gegenion
+
+
(M ) (M) (M ) (5.4)
9/14/2010 3:38:51 PM
K10478.indb 151
K10478.indb 151 9/14/2010 3:38:51 PM