Page 193 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 193
156 Carraher’s Polymer Chemistry
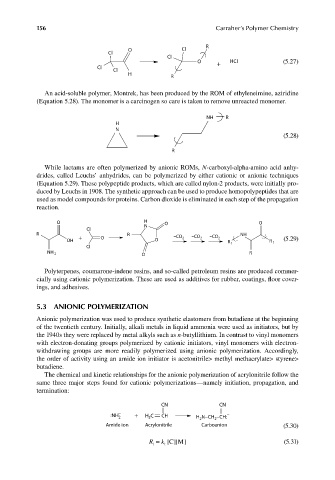
R
O Cl
Cl
Cl
O + HCI (5.27)
Cl
Cl
H
R
An acid-soluble polymer, Montrek, has been produced by the ROM of ethyleneimine, aziridine
(Equation 5.28). The monomer is a carcinogen so care is taken to remove unreacted monomer.
NH R
H
N
(5.28)
R
While lactams are often polymerized by anionic ROMs, N-carboxyl-alpha-amino acid anhy-
drides, called Leuchs’ anhydrides, can be polymerized by either cationic or anionic techniques
(Equation 5.29). These polypeptide products, which are called nylon-2 products, were initially pro-
duced by Leuchs in 1908. The synthetic approach can be used to produce homopolypeptides that are
used as model compounds for proteins. Carbon dioxide is eliminated in each step of the propagation
reaction.
O H O O
N
Cl
R R NH
OH + O O –CO 2 –CO 2 –CO 2 R 1 R 1 (5.29)
Cl
NH 2 R
O
Polyterpenes, coumarone-indene resins, and so-called petroleum resins are produced commer-
cially using cationic polymerization. These are used as additives for rubber, coatings, fl oor cover-
ings, and adhesives.
5.3 ANIONIC POLYMERIZATION
Anionic polymerization was used to produce synthetic elastomers from butadiene at the beginning
of the twentieth century. Initially, alkali metals in liquid ammonia were used as initiators, but by
the 1940s they were replaced by metal alkyls such as n-butyllithium. In contrast to vinyl monomers
with electron-donating groups polymerized by cationic initiators, vinyl monomers with electron-
withdrawing groups are more readily polymerized using anionic polymerization. Accordingly,
the order of activity using an amide ion initiator is acetonitrile> methyl methacrylate> styrene>
butadiene.
The chemical and kinetic relationships for the anionic polymerization of acrylonitrile follow the
same three major steps found for cationic polymerizations—namely initiation, propagation, and
termination:
CN CN
| |
−
:NH 2 + H C CH H 2 N−CH −CH: −
2
2
Amide ion Acrylonitrile Carboanion (5.30)
R = k [C][M] (5.31)
i
i
9/14/2010 3:38:57 PM
K10478.indb 156 9/14/2010 3:38:57 PM
K10478.indb 156