Page 195 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 195
158 Carraher’s Polymer Chemistry
is dependent on the dielectric constant of the solvent and the degree of solvation of the gegenion.
Weakly polar initiators, such as Grignard’s reagent, may be used when strong electron-withdrawing
groups are present on the monomer, but monomers with less electron-withdrawing groups require
more highly polar initiators such as n-butyllithium.
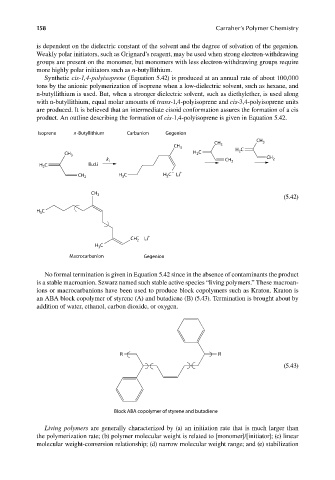
Synthetic cis-1,4-polyisoprene (Equation 5.42) is produced at an annual rate of about 100,000
tons by the anionic polymerization of isoprene when a low-dielectric solvent, such as hexane, and
n-butyllithium is used. But, when a stronger dielectric solvent, such as diethylether, is used along
with n-butyllithium, equal molar amounts of trans-1,4-polyisoprene and cis-3,4-polyisoprene units
are produced. It is believed that an intermediate cisoid conformation assures the formation of a cis
product. An outline describing the formation of cis-1,4-polyisoprene is given in Equation 5.42.
Isoprene n-Butyllithium Carbanion Gegenion
CH 3
CH 3
CH 3 H 2 C
H 2 C
CH 3
CH 2
k i CH 2
H 2 C Bu:Li
− +
H 3 C H 2 C Li
CH 2
CH 3
(5.42)
H 3 C
− +
CH 2 Li
H 3 C
Macrocarbanion Gegenion
No formal termination is given in Equation 5.42 since in the absence of contaminants the product
is a stable macroanion. Szwarz named such stable active species “living polymers.” These macroan-
ions or macrocarbanions have been used to produce block copolymers such as Kraton. Kraton is
an ABA block copolymer of styrene (A) and butadiene (B) (5.43). Termination is brought about by
addition of water, ethanol, carbon dioxide, or oxygen.
R R
(5.43)
Block ABA copolymer of styrene and butadiene
Living polymers are generally characterized by (a) an initiation rate that is much larger than
the polymerization rate; (b) polymer molecular weight is related to [monomer]/[initiator]; (c) linear
molecular weight-conversion relationship; (d) narrow molecular weight range; and (e) stabilization
9/14/2010 3:38:59 PM
K10478.indb 158
K10478.indb 158 9/14/2010 3:38:59 PM