Page 196 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 196
Ionic Chain-Reaction and Complex Coordination Polymerization 159
of the living end groups allowing the formation of telechelics, macromers, block copolymers, and
star polymers.
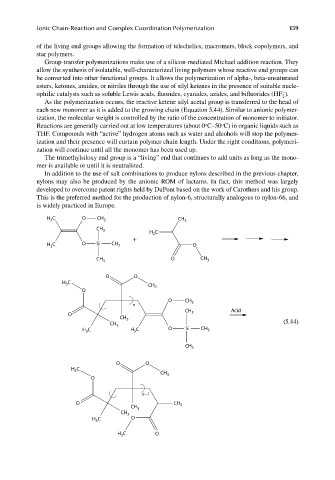
Group-transfer polymerizations make use of a silicon-mediated Michael addition reaction. They
allow the synthesis of isolatable, well-characterized living polymers whose reactive end groups can
be converted into other functional groups. It allows the polymerization of alpha-, beta-unsaturated
esters, ketones, amides, or nitriles through the use of silyl ketenes in the presence of suitable nucle-
−
ophilic catalysts such as soluble Lewis acids, fluorides, cyanides, azides, and bifl uorides (HF ).
2
As the polymerization occurs, the reactive ketene silyl acetal group is transferred to the head of
each new monomer as it is added to the growing chain (Equation 5.44). Similar to anionic polymer-
ization, the molecular weight is controlled by the ratio of the concentration of monomer to initiator.
o
o
Reactions are generally carried out at low temperatures (about 0 C–50 C) in organic liquids such as
THF. Compounds with “active” hydrogen atoms such as water and alcohols will stop the polymer-
ization and their presence will curtain polymer chain length. Under the right conditions, polymeri-
zation will continue until all the monomer has been used up.
The trimethylsiloxy end group is a “living” end that continues to add units as long as the mono-
mer is available or until it is neutralized.
In addition to the use of salt combinations to produce nylons described in the previous chapter,
nylons may also be produced by the anionic ROM of lactams. In fact, this method was largely
developed to overcome patent rights held by DuPont based on the work of Carothers and his group.
This is the preferred method for the production of nylon-6, structurally analogous to nylon-66, and
is widely practiced in Europe.
H C O CH 3 CH 3
3
CH 3
H C
2
+
H C O Si CH 3 O
3
CH 3 O CH 3
O O
C
H 3
CH 3
O
O CH
n 3
CH 3 Acid
O
CH 3
CH 3 (5.44)
C H C O Si CH
H 3 3 3
CH 3
O O
H 3 C
CH 3
O
n−1
O CH
CH 3 3
CH 3
H C O
3
H C O
3
9/14/2010 3:39:00 PM
K10478.indb 159 9/14/2010 3:39:00 PM
K10478.indb 159