Page 249 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 249
212 Carraher’s Polymer Chemistry
Poly(vinyl chloride) has a built in advantage over many other polymers in that it is itself fl ame
resistant. About 50% of PVC is used as rigid pipe. About 70% of the water pipes in the United
States are PVC. About 75% of the sewer pipes are PVC. Other uses of rigid PVC are as pipe fi t-
tings, electrical outlet boxes, and automotive parts. Uses of flexible PVC include gasoline-resistant
hose, hospital sheeting, shoe soles, electrical tape, stretch film, pool liners, vinyl-coated fabrics,
roof coatings, refrigerator gaskets, floor sheeting, and electrical insulation and jacketing. A wide
number of vinyl chloride copolymers are commercially used. Many vinyl floor tiles are copoly-
mers of PVC.
Many flat sheet signs are made of PVC. Films are also formed from PVC. Many of these signs
and films are simply referred to as vinyl. These films and sheets form many of our commercial signs
and markings on vehicles. Unplasticized or rigid PVC is used in the construction industry as a siding
simply known as vinyl siding. It is also used to repair window frames and sills and fascia. It is also
widely used in the construction of plastic gutters, downpipes, and drainpipes.
The flame resistance of PVC is a mixed blessing. In a fire, the PVC emits hydrogen chloride with
the chlorine scavenging free radicals helping eliminate the fire. Hydrogen chloride fumes present a
health concern when we breathe them. In fire, moisture helps dilute the hydrogen chloride causing
it to settle on the cooler surfaces rather than remaining air borne. Even so, in closed structures such
as tunnels alternative materials are advised.
Table 6.9 contains general physical properties of PVC. Because of the variety of additives, the
values for the plasticized PVC are approximate.
Poly(vinyl chloride) is flexibilized by addition of plasticizers as already noted. It is also made
more flexible through blending it with elastomers that act as impact modifiers. These blends are
used when impact resistance is essential.
A number of copolymers are formed employing vinyl chloride. Because of the presence of the
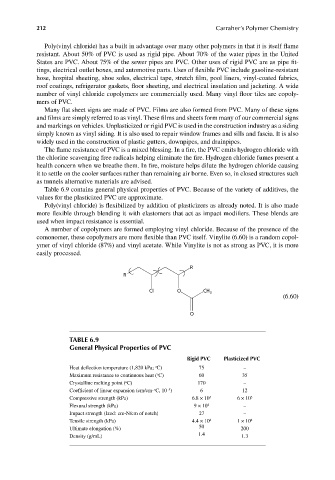
comonomer, these copolymers are more flexible than PVC itself. Vinylite (6.60) is a random copol-
ymer of vinyl chloride (87%) and vinyl acetate. While Vinylite is not as strong as PVC, it is more
easily processed.
R
R
Cl O CH 3
(6.60)
O
TABLE 6.9
General Physical Properties of PVC
Rigid PVC Plasticized PVC
Heat deflection temperature (1,820 kPa; C) 75 –
o
o
Maximum resistance to continuous heat ( C) 60 35
o
Crystalline melting point ( C) 170 –
–5
o
Coefficient of linear expansion (cm/cm- C, 10 ) 6 12
Compressive strength (kPa) 6.8 × 10 4 6 × 10 3
Flexural strength (kPa) 9 × 10 4 –
Impact strength (Izod: cm-N/cm of notch) 27 –
Tensile strength (kPa) 4.4 × 10 4 1 × 10 4
50
Ultimate elongation (%) 200
Density (g/mL) 1.4 1.3
9/14/2010 3:39:41 PM
K10478.indb 212 9/14/2010 3:39:41 PM
K10478.indb 212