Page 253 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 253
216 Carraher’s Polymer Chemistry
acetal rings on these random amorphous polymer chains restrict flexibility and increase the heat
deflection temperature to a value higher than that of PVAc.
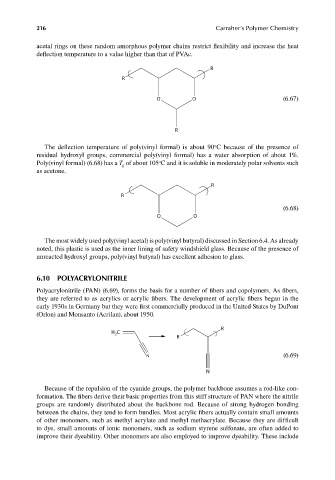
R
R
O O (6.67)
R
o
The deflection temperature of poly(vinyl formal) is about 90 C because of the presence of
residual hydroxyl groups, commercial poly(vinyl formal) has a water absorption of about 1%.
o
Poly(vinyl formal) (6.68) has a T of about 105 C and it is soluble in moderately polar solvents such
g
as acetone.
R
R
(6.68)
O O
The most widely used poly(vinyl acetal) is poly(vinyl butyral) discussed in Section 6.4. As already
noted, this plastic is used as the inner lining of safety windshield glass. Because of the presence of
unreacted hydroxyl groups, poly(vinyl butyral) has excellent adhesion to glass.
6.10 POLYACRYLONITRILE
Polyacrylonitrile (PAN) (6.69), forms the basis for a number of fibers and copolymers. As fi bers,
they are referred to as acrylics or acrylic fibers. The development of acrylic fibers began in the
early 1930s in Germany but they were first commercially produced in the United States by DuPont
(Orlon) and Monsanto (Acrilan), about 1950.
R
H C
2
R
N (6.69)
N
Because of the repulsion of the cyanide groups, the polymer backbone assumes a rod-like con-
formation. The fibers derive their basic properties from this stiff structure of PAN where the nitrile
groups are randomly distributed about the backbone rod. Because of strong hydrogen bonding
between the chains, they tend to form bundles. Most acrylic fi bers actually contain small amounts
of other monomers, such as methyl acrylate and methyl methacrylate. Because they are diffi cult
to dye, small amounts of ionic monomers, such as sodium styrene sulfonate, are often added to
improve their dyeability. Other monomers are also employed to improve dyeability. These include
9/14/2010 3:39:45 PM
K10478.indb 216
K10478.indb 216 9/14/2010 3:39:45 PM