Page 254 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 254
Free Radical Chain Polymerization 217
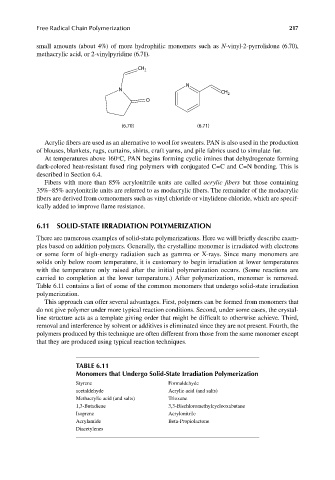
small amounts (about 4%) of more hydrophilic monomers such as N-vinyl-2-pyrrolidone (6.70),
methacrylic acid, or 2-vinylpyridine (6.71).
CH 2
N
N
CH 2
O
(6.70) (6.71)
Acrylic fibers are used as an alternative to wool for sweaters. PAN is also used in the production
of blouses, blankets, rugs, curtains, shirts, craft yarns, and pile fabrics used to simulate fur.
At temperatures above 160 C, PAN begins forming cyclic imines that dehydrogenate forming
o
dark-colored heat-resistant fused ring polymers with conjugated C=C and C=N bonding. This is
described in Section 6.4.
Fibers with more than 85% acrylonitrile units are called acrylic fi bers but those containing
35%–85% acrylonitrile units are referred to as modacrylic fibers. The remainder of the modacrylic
fibers are derived from comonomers such as vinyl chloride or vinylidene chloride, which are specif-
ically added to improve fl ame resistance.
6.11 SOLID-STATE IRRADIATION POLYMERIZATION
There are numerous examples of solid-state polymerizations. Here we will briefly describe exam-
ples based on addition polymers. Generally, the crystalline monomer is irradiated with electrons
or some form of high-energy radiation such as gamma or X-rays. Since many monomers are
solids only below room temperature, it is customary to begin irradiation at lower temperatures
with the temperature only raised after the initial polymerization occurs. (Some reactions are
carried to completion at the lower temperature.) After polymerization, monomer is removed.
Table 6.11 contains a list of some of the common monomers that undergo solid-state irradiation
polymerization.
This approach can offer several advantages. First, polymers can be formed from monomers that
do not give polymer under more typical reaction conditions. Second, under some cases, the crystal-
line structure acts as a template giving order that might be difficult to otherwise achieve. Third,
removal and interference by solvent or additives is eliminated since they are not present. Fourth, the
polymers produced by this technique are often different from those from the same monomer except
that they are produced using typical reaction techniques.
TABLE 6.11
Monomers that Undergo Solid-State Irradiation Polymerization
Styrene Formaldehyde
acetaldehyde Acrylic acid (and salts)
Methacrylic acid (and salts) Trioxane
1,3-Butadiene 3,3-Bischloromethylcyclooxabutane
Isoprene Acrylonitrile
Acrylamide Beta-Propiolactone
Diacetylenes
9/14/2010 3:39:46 PM
K10478.indb 217 9/14/2010 3:39:46 PM
K10478.indb 217