Page 305 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 305
268 Carraher’s Polymer Chemistry
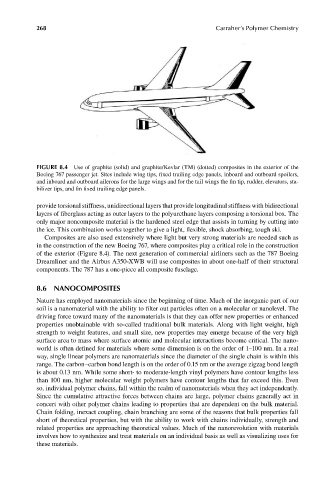
FIGURE 8.4 Use of graphite (solid) and graphite/Kevlar (TM) (dotted) composites in the exterior of the
Boeing 767 passenger jet. Sites include wing tips, fixed trailing edge panels, inboard and outboard spoilers,
and inboard and outboard ailerons for the large wings and for the tail wings the fin tip, rudder, elevators, sta-
bilizer tips, and fi n fixed trailing edge panels.
provide torsional stiffness, unidirectional layers that provide longitudinal stiffness with bidirectional
layers of fiberglass acting as outer layers to the polyurethane layers composing a torsional box. The
only major noncomposite material is the hardened steel edge that assists in turning by cutting into
the ice. This combination works together to give a light, flexible, shock absorbing, tough ski.
Composites are also used extensively where light but very strong materials are needed such as
in the construction of the new Boeing 767, where composites play a critical role in the construction
of the exterior (Figure 8.4). The next generation of commercial airliners such as the 787 Boeing
Dreamliner and the Airbus A350-XWB will use composites in about one-half of their structural
components. The 787 has a one-piece all composite fuselage.
8.6 NANOCOMPOSITES
Nature has employed nanomaterials since the beginning of time. Much of the inorganic part of our
soil is a nanomaterial with the ability to fi lter out particles often on a molecular or nanolevel. The
driving force toward many of the nanomaterials is that they can offer new properties or enhanced
properties unobtainable with so-called traditional bulk materials. Along with light weight, high
strength to weight features, and small size, new properties may emerge because of the very high
surface area to mass where surface atomic and molecular interactions become critical. The nano-
world is often defined for materials where some dimension is on the order of 1–100 nm. In a real
way, single linear polymers are nanomaterials since the diameter of the single chain is within this
range. The carbon–carbon bond length is on the order of 0.15 nm or the average zigzag bond length
is about 0.13 nm. While some short- to moderate-length vinyl polymers have contour lengths less
than 100 nm, higher molecular weight polymers have contour lengths that far exceed this. Even
so, individual polymer chains, fall within the realm of nanomaterials when they act independently.
Since the cumulative attractive forces between chains are large, polymer chains generally act in
concert with other polymer chains leading to properties that are dependent on the bulk material.
Chain folding, inexact coupling, chain branching are some of the reasons that bulk properties fall
short of theoretical properties, but with the ability to work with chains individually, strength and
related properties are approaching theoretical values. Much of the nanorevolution with materials
involves how to synthesize and treat materials on an individual basis as well as visualizing uses for
these materials.
9/14/2010 3:40:29 PM
K10478.indb 268 9/14/2010 3:40:29 PM
K10478.indb 268