Page 320 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 320
Naturally Occurring Polymers—Plants 283
Strong basic solutions, such as sodium hydroxide, penetrate the crystalline lattice of α-cellulose,
producing an alkoxide called alkali or soda cellulose. Mercerized cotton is produced by aqueous
extraction of the sodium hydroxide.
Most linear celluloses may be dissolved in solvents capable of breaking the strong hydrogen
bonds. These solutions include aqueous solutions of inorganic acids, calcium thiocyanate, zinc chlo-
ride, lithium chloride, ammonium hydroxide, iron sodium tartrate, and cadmium or copper ammo-
nium hydroxide (Schweitzer’s reagent). The product precipitated by the addition of a nonsolvent to
these solutions is highly amorphous regenerated cellulose.
OH OH OH OH
H O O H O O H H O O H H O R
H H H OH H OH H
OH H OH H H
R H H (9.1)
H OH H OH H OH H OH
Cellulose
H OH
H OH
H
OH OH H OH H
H OH H
H O O H
H O O H O O H O
H H R (9.2)
R OH H OH OH H OH
H H
H OH H OH
Cellulose 3D structure
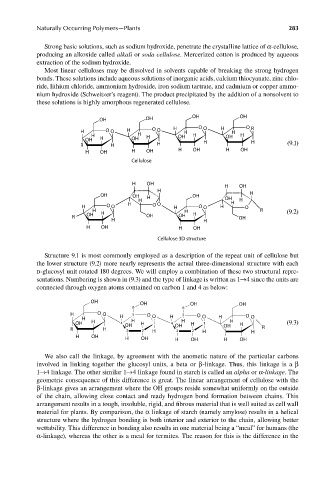
Structure 9.1 is most commonly employed as a description of the repeat unit of cellulose but
the lower structure (9.2) more nearly represents the actual three-dimensional structure with each
d-glucosyl unit rotated 180 degrees. We will employ a combination of these two structural repre-
sentations. Numbering is shown in (9.3) and the type of linkage is written as 1→4 since the units are
connected through oxygen atoms contained on carbon 1 and 4 as below:
OH OH OH OH
6 6
H O O H 5 O O 5 O O O O
H H H
OH H 4 OH H H 1 4 OH H H 1 OH H H (9.3)
R H 3 2 H 3 2 H H R
H OH H OH
H OH H OH
We also call the linkage, by agreement with the anometic nature of the particular carbons
involved in linking together the glucosyl units, a beta or β-linkage. Thus, this linkage is a β
1→4 linkage. The other similar 1→4 linkage found in starch is called an alpha or α-linkage. The
geometric consequence of this difference is great. The linear arrangement of cellulose with the
β-linkage gives an arrangement where the OH groups reside somewhat uniformly on the outside
of the chain, allowing close contact and ready hydrogen bond formation between chains. This
arrangement results in a tough, insoluble, rigid, and fibrous material that is well suited as cell wall
material for plants. By comparison, the α linkage of starch (namely amylose) results in a helical
structure where the hydrogen bonding is both interior and exterior to the chain, allowing better
wettability. This difference in bonding also results in one material being a “meal” for humans (the
α-linkage), whereas the other is a meal for termites. The reason for this is the difference in the
9/14/2010 3:40:38 PM
K10478.indb 283 9/14/2010 3:40:38 PM
K10478.indb 283