Page 465 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 465
428 Carraher’s Polymer Chemistry
OH –
– O
O – –
O O O O – O OH
OH Si Si OH O Al Al Si
Al
O O
O O O
O
Si Al
Si OH Al Al Si
OH O O OH OH O O –
OH O –
– –
O O
(a) (b) (c)
–
O O –
–
– OH O
O O OH OH OH
Si O O
Al O – Si Al Al Si
O O
O O
O O
Si –
Si O Al Si O –
OH O Al Al O
OH O OH –
– O OH
O O –
(d) (e) (f)
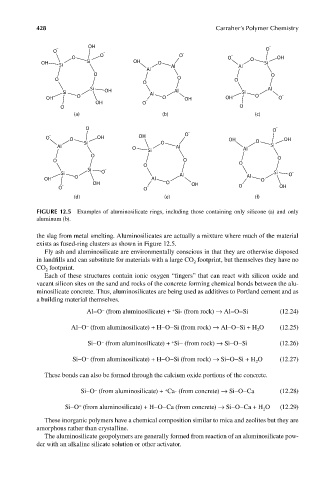
FIGURE 12.5 Examples of aluminosilicate rings, including those containing only silicone (a) and only
aluminum (b).
the slag from metal smelting. Aluminosilicates are actually a mixture where much of the material
exists as fused-ring clusters as shown in Figure 12.5.
Fly ash and aluminosilicate are environmentally conscious in that they are otherwise disposed
in landfills and can substitute for materials with a large CO footprint, but themselves they have no
2
CO footprint.
2
Each of these structures contain ionic oxygen “fingers” that can react with silicon oxide and
vacant silicon sites on the sand and rocks of the concrete forming chemical bonds between the alu-
minosilicate concrete. Thus, aluminosilicates are being used as additives to Portland cement and as
a building material themselves.
Al–O (from aluminosilicate) + Si- (from rock) → Al–O–Si (12.24)
−
+
Al–O (from aluminosilicate) + H–O–Si (from rock) → Al–O–Si + H O (12.25)
−
2
+
Si–O (from aluminosilicate) + Si– (from rock) → Si–O–Si (12.26)
−
Si–O (from aluminosilicate) + H–O–Si (from rock) → Si–O–Si + H O (12.27)
−
2
These bonds can also be formed through the calcium oxide portions of the concrete.
−
Si–O (from aluminosilicate) + Ca- (from concrete) → Si–O–Ca (12.28)
+
Si–O (from aluminosilicate) + H–O–Ca (from concrete) → Si–O–Ca + H O (12.29)
−
2
These inorganic polymers have a chemical composition similar to mica and zeolites but they are
amorphous rather than crystalline.
The aluminosilicate geopolymers are generally formed from reaction of an aluminosilicate pow-
der with an alkaline silicate solution or other activator.
9/14/2010 3:42:04 PM
K10478.indb 428
K10478.indb 428 9/14/2010 3:42:04 PM