Page 469 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 469
432 Carraher’s Polymer Chemistry
R
H C H 2 C H 2 C H C H C H 2 C H 2 C H 2 C H C H C
2
2
2
2
2
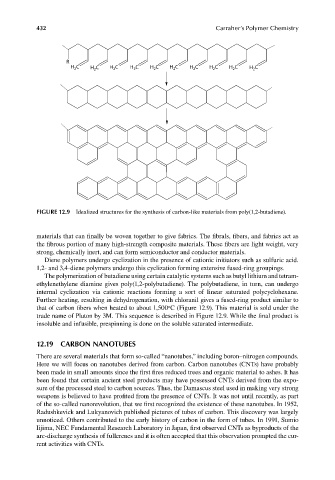
FIGURE 12.9 Idealized structures for the synthesis of carbon-like materials from poly(1,2-butadiene).
materials that can finally be woven together to give fabrics. The fi brals, fibers, and fabrics act as
the fibrous portion of many high-strength composite materials. These fibers are light weight, very
strong, chemically inert, and can form semiconductor and conductor materials.
Diene polymers undergo cyclization in the presence of cationic initiators such as sulfuric acid.
1,2- and 3,4-diene polymers undergo this cyclization forming extensive fused-ring groupings.
The polymerization of butadiene using certain catalytic systems such as butyl lithium and tetram-
ethylenethylene diamine gives poly(1,2-polybutadiene). The polybutadiene, in turn, can undergo
internal cyclization via cationic reactions forming a sort of linear saturated polycyclohexane.
Further heating, resulting in dehydrogenation, with chloranil gives a fused-ring product similar to
that of carbon fibers when heated to about 1,500 C (Figure 12.9). This material is sold under the
o
trade name of Pluton by 3M. This sequence is described in Figure 12.9. While the fi nal product is
insoluble and infusible, prespinning is done on the soluble saturated intermediate.
12.19 CARBON NANOTUBES
There are several materials that form so-called “nanotubes,” including boron–nitrogen compounds.
Here we will focus on nanotubes derived from carbon. Carbon nanotubes (CNTs) have probably
been made in small amounts since the fi rst fires reduced trees and organic material to ashes. It has
been found that certain ancient steel products may have possessed CNTs derived from the expo-
sure of the processed steel to carbon sources. Thus, the Damascus steel used in making very strong
weapons is believed to have profited from the presence of CNTs. It was not until recently, as part
of the so-called nanorevolution, that we first recognized the existence of these nanotubes. In 1952,
Radushkevick and Lukyanovich published pictures of tubes of carbon. This discovery was largely
unnoticed. Others contributed to the early history of carbon in the form of tubes. In 1991, Sumio
Iijima, NEC Fundamental Research Laboratory in Japan, first observed CNTs as byproducts of the
arc-discharge synthesis of fullerenes and it is often accepted that this observation prompted the cur-
rent activities with CNTs.
9/14/2010 3:42:06 PM
K10478.indb 432 9/14/2010 3:42:06 PM
K10478.indb 432