Page 466 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 466
Inorganic Polymers 429
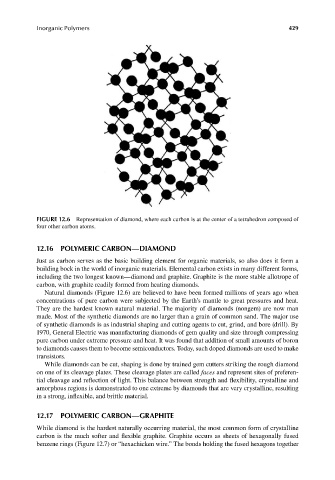
FIGURE 12.6 Representation of diamond, where each carbon is at the center of a tetrahedron composed of
four other carbon atoms.
12.16 POLYMERIC CARBON—DIAMOND
Just as carbon serves as the basic building element for organic materials, so also does it form a
building bock in the world of inorganic materials. Elemental carbon exists in many different forms,
including the two longest known—diamond and graphite. Graphite is the more stable allotrope of
carbon, with graphite readily formed from heating diamonds.
Natural diamonds (Figure 12.6) are believed to have been formed millions of years ago when
concentrations of pure carbon were subjected by the Earth’s mantle to great pressures and heat.
They are the hardest known natural material. The majority of diamonds (nongem) are now man
made. Most of the synthetic diamonds are no larger than a grain of common sand. The major use
of synthetic diamonds is as industrial shaping and cutting agents to cut, grind, and bore (drill). By
1970, General Electric was manufacturing diamonds of gem quality and size through compressing
pure carbon under extreme pressure and heat. It was found that addition of small amounts of boron
to diamonds causes them to become semiconductors. Today, such doped diamonds are used to make
transistors.
While diamonds can be cut, shaping is done by trained gem cutters striking the rough diamond
on one of its cleavage plates. These cleavage plates are called faces and represent sites of preferen-
tial cleavage and reflection of light. This balance between strength and flexibility, crystalline and
amorphous regions is demonstrated to one extreme by diamonds that are very crystalline, resulting
in a strong, inflexible, and brittle material.
12.17 POLYMERIC CARBON—GRAPHITE
While diamond is the hardest naturally occurring material, the most common form of crystalline
carbon is the much softer and flexible graphite. Graphite occurs as sheets of hexagonally fused
benzene rings (Figure 12.7) or “hexachicken wire.” The bonds holding the fused hexagons together
9/14/2010 3:42:04 PM
K10478.indb 429
K10478.indb 429 9/14/2010 3:42:04 PM