Page 552 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 552
Additives 515
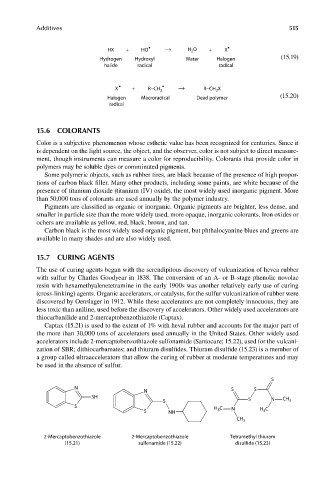
HX + HO ⋅ H O + X ⋅
2
(15.19)
Hydrogen Hydroxyl Water Halogen
halide radical radical
X + R−CH R−CH X
2
2
(15.20)
Halogen Macroradical Dead polymer
radical
15.6 COLORANTS
Color is a subjective phenomenon whose esthetic value has been recognized for centuries. Since it
is dependent on the light source, the object, and the observer, color is not subject to direct measure-
ment, though instruments can measure a color for reproducibility. Colorants that provide color in
polymers may be soluble dyes or comminuted pigments.
Some polymeric objects, such as rubber tires, are black because of the presence of high propor-
tions of carbon black filler. Many other products, including some paints, are white because of the
presence of titanium dioxide (titanium (IV) oxide), the most widely used inorganic pigment. More
than 50,000 tons of colorants are used annually by the polymer industry.
Pigments are classified as organic or inorganic. Organic pigments are brighter, less dense, and
smaller in particle size than the more widely used, more opaque, inorganic colorants. Iron oxides or
ochers are available as yellow, red, black, brown, and tan.
Carbon black is the most widely used organic pigment, but phthalocyanine blues and greens are
available in many shades and are also widely used.
15.7 CURING AGENTS
The use of curing agents began with the serendipitous discovery of vulcanization of hevea rubber
with sulfur by Charles Goodyear in 1838. The conversion of an A- or B-stage phenolic novolac
resin with hexamethyalenetetramine in the early 1900s was another relatively early use of curing
(cross-linking) agents. Organic accelerators, or catalysts, for the sulfur vulcanization of rubber were
discovered by Oenslager in 1912. While these accelerators are not completely innocuous, they are
less toxic than aniline, used before the discovery of accelerators. Other widely used accelerators are
thiocarbanilide and 2-mercaptobenzothiazole (Captax).
Captax (15.21) is used to the extent of 1% with heval rubber and accounts for the major part of
the more than 30,000 tons of accelerators used annually in the United States. Other widely used
accelerators include 2-mercaptobenzothiazole sulfonamide (Santocure; 15.22), used for the vulcani-
zation of SBR; dithiocarbamates; and thiuram disulfi des. Thiuram disulfide (15.23) is a member of
a group called ultraaccelerators that allow the curing of rubber at moderate temperatures and may
be used in the absence of sulfur.
S
N S S
N
SH S N CH
S 3
S H C
3
S NH 3 N H C
CH 3
2-Mercaptobenzothiazole 2-Mercaptobenzothiazole Tetramethyl thiuram
(15.21) sulfenamide (15.22) disulfide (15.23)
9/14/2010 3:42:49 PM
K10478.indb 515 9/14/2010 3:42:49 PM
K10478.indb 515