Page 553 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 553
516 Carraher’s Polymer Chemistry
Initiators such as benzoyl peroxide are used not only for the initiation of chain-reaction poly-
merization, but also for the curing of polyesters and ethylene–propylene copolymers, and for the
grafting of styrene on elastomeric polymer chains.
Unsaturated polymers such as alkyd resins can be cured or “dried” in the presence of oxygen, a
heavy metal, and an organic acid called a drier. The most common organic acids are linoleic, abi-
etic, naphthenic, octoic, and tall oil fatty acids.
15.8 ANTISTATIC AGENTS—ANTISTATS
Antistatic agents (antistats) dissipate static electrical charges. Insulating materials, including most
organic plastics, fi bers, films, and elastomers, can build up electrical charge. Because these largely
organic materials are insulators, they are not able to dissipate the charge. Such charge buildup is
particularly noticeable in cold, dry climates and lead to dust attraction and sparking.
Antistatic agents can be either internal or external. External antistats are applied to the surface by
wiping, spraying, and so on. These surface treatments act to prevent static charge buildup. Internal
antistats are added during the processing and become an integral part of the bulk composition of the
material. Because surface treatments are often worn away through washing, waxing, and handling,
the external antistats must be replenished. Internal antistats are added to allow the antistats to come
(“bleed”) to the surface over a long time, giving the material long-term protection. Many antistatic
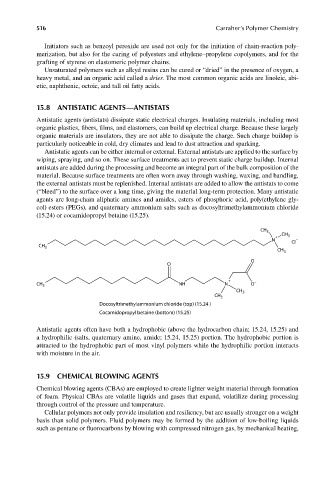
agents are long-chain aliphatic amines and amides, esters of phosphoric acid, poly(ethylene gly-
col) esters (PEGs), and quaternary ammonium salts such as docosyltrimethylammonium chloride
(15.24) or cocamidopropyl betaine (15.25).
CH 3
+ CH 3
N Cl –
CH 3
CH 3
O
O
+ –
NH N O
CH 3
CH 3
CH 3
Docosyltrimethylammonium chloride (top) (15.24 )
Cocamidopropyl betaine (bottom) (15.25)
Antistatic agents often have both a hydrophobic (above the hydrocarbon chain; 15.24, 15.25) and
a hydrophilic (salts, quaternary amine, amide; 15.24, 15.25) portion. The hydrophobic portion is
attracted to the hydrophobic part of most vinyl polymers while the hydrophilic portion interacts
with moisture in the air.
15.9 CHEMICAL BLOWING AGENTS
Chemical blowing agents (CBAs) are employed to create lighter weight material through formation
of foam. Physical CBAs are volatile liquids and gases that expand, volatilize during processing
through control of the pressure and temperature.
Cellular polymers not only provide insulation and resiliency, but are usually stronger on a weight
basis than solid polymers. Fluid polymers may be formed by the addition of low-boiling liquids
such as pentane or fluorocarbons by blowing with compressed nitrogen gas, by mechanical heating,
9/14/2010 3:42:49 PM
K10478.indb 516 9/14/2010 3:42:49 PM
K10478.indb 516