Page 550 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 550
Additives 513
hydrogen chloride forming chromophoric conjugated polyene structures eventually yielding fused
aromatic ring systems.
This type of degradation is accelerated in the presence of iron salts, oxygen, and hydrogen chlo-
ride. Toxic lead and barium and cadmium salts act as scavengers for hydrogen chloride and may
be used for some applications such as wire coating. Mixtures of magnesium and calcium stearates
are less toxic and effective. Among the most widely used heat stabilizers for PVC are the organotin
products. Many of these are based on organotin polymers discovered by Carraher.
R
R HCl + R R
(15.15)
Cl Cl Cl Cl Cl Cl
15.4 UV STABILIZERS
Ultraviolet stabilizers act to quench UV radiation. While much of the sun’s high-energy radiation
is absorbed by the atmosphere, some radiation in the 280–400 nm (UV) range reaches the earth’s
surface. Since the energy of this radiation is in the order of 70–100 kcal/mol (280–400 kJ/mol), UV
radiation is sufficient to break many chemical bonds, and thus cause polymer chains to break. This
breakage generally does one of two things: (1) it can increase the amount of cross-linking through
subsequent formation of bonds after the initial bond breakage; or (2) bond breakage can result in a
decreased chain length. Either of these results in a decreased overall strength of the polymeric mate-
rial, and often the yellowing of many organic polymers. Cross-linking also results in the embrittle-
ment of these polymers.
Poly(ethylene) PE, PVC, polystyrene (PS), poly(ethylene terephthalate) (PET), and PP are
degraded at wavelengths of 300, 310, 319, 325, and 370 nm, respectively. The bond energy required
to cleave the tertiary carbon hydrogen bond in PP is only 320 kJ/mol. This corresponds to a wave-
length of 318 nm.
Since the effect of UV radiation on synthetic polymers is similar to its effect on the human skin,
it is not surprising that UV stabilizers such as phenyl salicylate have been used for commercial poly-
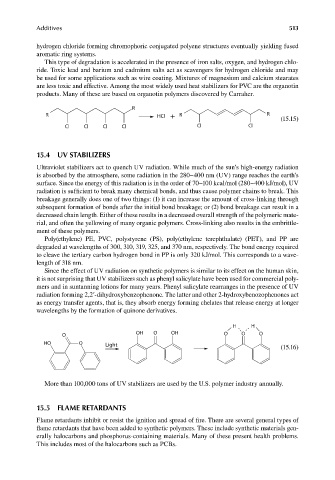
mers and in suntanning lotions for many years. Phenyl salicylate rearranges in the presence of UV
radiation forming 2,2′-dihydroxybenzophenone. The latter and other 2-hydroxybenozophenones act
as energy transfer agents, that is, they absorb energy forming chelates that release energy at longer
wavelengths by the formation of quinone derivatives.
H H
OH O OH O O O
O
HO O Light
(15.16)
More than 100,000 tons of UV stabilizers are used by the U.S. polymer industry annually.
15.5 FLAME RETARDANTS
Flame retardants inhibit or resist the ignition and spread of fire. There are several general types of
flame retardants that have been added to synthetic polymers. These include synthetic materials gen-
erally halocarbons and phosphorus-containing materials. Many of these present health problems.
This includes most of the halocarbons such as PCBs.
9/14/2010 3:42:46 PM
K10478.indb 513
K10478.indb 513 9/14/2010 3:42:46 PM