Page 586 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 586
Reactions on Polymers 549
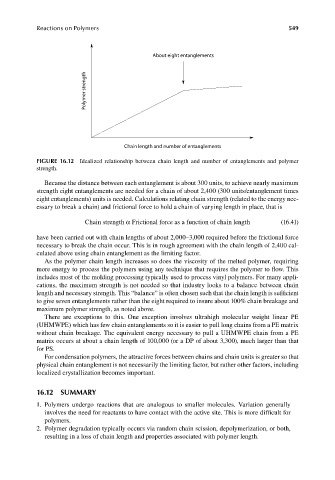
About eight entanglements
Polymer strength
Chain length and number of entanglements
FIGURE 16.12 Idealized relationship between chain length and number of entanglements and polymer
strength.
Because the distance between each entanglement is about 300 units, to achieve nearly maximum
strength eight entanglements are needed for a chain of about 2,400 (300 units/entanglement times
eight entanglements) units is needed. Calculations relating chain strength (related to the energy nec-
essary to break a chain) and frictional force to hold a chain of varying length in place, that is
Chain strength α Frictional force as a function of chain length (16.41)
have been carried out with chain lengths of about 2,000–3,000 required before the frictional force
necessary to break the chain occur. This is in rough agreement with the chain length of 2,400 cal-
culated above using chain entanglement as the limiting factor.
As the polymer chain length increases so does the viscosity of the melted polymer, requiring
more energy to process the polymers using any technique that requires the polymer to fl ow. This
includes most of the molding processing typically used to process vinyl polymers. For many appli-
cations, the maximum strength is not needed so that industry looks to a balance between chain
length and necessary strength. This “balance” is often chosen such that the chain length is suffi cient
to give seven entanglements rather than the eight required to insure about 100% chain breakage and
maximum polymer strength, as noted above.
There are exceptions to this. One exception involves ultrahigh molecular weight linear PE
(UHMWPE) which has few chain entanglements so it is easier to pull long chains from a PE matrix
without chain breakage. The equivalent energy necessary to pull a UHMWPE chain from a PE
matrix occurs at about a chain length of 100,000 (or a DP of about 3,300), much larger than that
for PS.
For condensation polymers, the attractive forces between chains and chain units is greater so that
physical chain entanglement is not necessarily the limiting factor, but rather other factors, including
localized crystallization becomes important.
16.12 SUMMARY
1. Polymers undergo reactions that are analogous to smaller molecules. Variation generally
involves the need for reactants to have contact with the active site. This is more diffi cult for
polymers.
2. Polymer degradation typically occurs via random chain scission, depolymerization, or both,
resulting in a loss of chain length and properties associated with polymer length.
9/14/2010 3:43:16 PM
K10478.indb 549 9/14/2010 3:43:16 PM
K10478.indb 549