Page 73 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 73
36 Carraher’s Polymer Chemistry
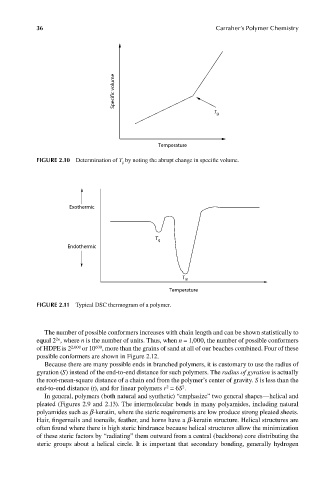
Specific volume
T g
Temperature
FIGURE 2.10 Determination of T g by noting the abrupt change in specifi c volume.
Exothermic
T g
Endothermic
T m
Temperature
FIGURE 2.11 Typical DSC thermogram of a polymer.
The number of possible conformers increases with chain length and can be shown statistically to
2n
equal 2 , where n is the number of units. Thus, when n = 1,000, the number of possible conformers
of HDPE is 2 2,000 or 10 , more than the grains of sand at all of our beaches combined. Four of these
600
possible conformers are shown in Figure 2.12.
Because there are many possible ends in branched polymers, it is customary to use the radius of
gyration (S) instead of the end-to-end distance for such polymers. The radius of gyration is actually
the root-mean-square distance of a chain end from the polymer’s center of gravity. S is less than the
2
end-to-end distance (r), and for linear polymers r = 6S .
2
In general, polymers (both natural and synthetic) “emphasize” two general shapes—helical and
pleated (Figures 2.9 and 2.13). The intermolecular bonds in many polyamides, including natural
polyamides such as β-keratin, where the steric requirements are low produce strong pleated sheets.
Hair, fingernails and toenails, feather, and horns have a β-keratin structure. Helical structures are
often found where there is high steric hindrance because helical structures allow the minimization
of these steric factors by “radiating” them outward from a central (backbone) core distributing the
steric groups about a helical circle. It is important that secondary bonding, generally hydrogen
9/14/2010 3:35:57 PM
K10478.indb 36 9/14/2010 3:35:57 PM
K10478.indb 36