Page 68 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 68
Polymer Structure (Morphology) 31
R R
CH 3
CH 3 CH 3
H 3 C
CH 3
CH 3 CH 3
CH 3
CH 3
CH 3 CH 3 R
H 3 C
H 3 C CH 3
H 3 C
CH 3 CH 3 CH 3
CH 3
CH 3
CH 3 CH 3
CH 3 CH 3 CH 3
CH 3
CH 3
CH 3 CH 3
H 3 C H 3 C CH 3
H 3 C CH 3
CH 3 CH 3
H 3 C H 3 C
H 3 C
CH 3 CH 3
R
CH 3
H 3 C
CH 3 CH 3
(a) (b)
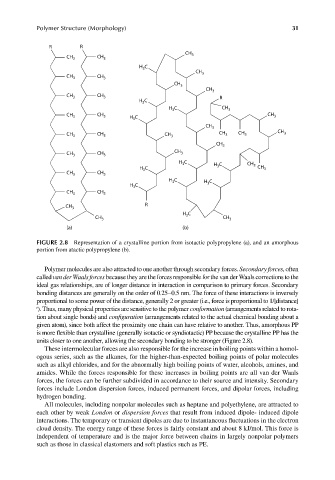
FIGURE 2.8 Representation of a crystalline portion from isotactic polypropylene (a), and an amorphous
portion from atactic polypropylene (b).
Polymer molecules are also attracted to one another through secondary forces. Secondary forces, often
called van der Waals forces because they are the forces responsible for the van der Waals corrections to the
ideal gas relationships, are of longer distance in interaction in comparison to primary forces. Secondary
bonding distances are generally on the order of 0.25–0.5 nm. The force of these interactions is inversely
proportional to some power of the distance, generally 2 or greater (i.e., force is proportional to 1/[distance]
r ). Thus, many physical properties are sensitive to the polymer conformation (arrangements related to rota-
tion about single bonds) and confi guration (arrangements related to the actual chemical bonding about a
given atom), since both affect the proximity one chain can have relative to another. Thus, amorphous PP
is more flexible than crystalline (generally isotactic or syndiotactic) PP because the crystalline PP has the
units closer to one another, allowing the secondary bonding to be stronger (Figure 2.8).
These intermolecular forces are also responsible for the increase in boiling points within a homol-
ogous series, such as the alkanes, for the higher-than-expected boiling points of polar molecules
such as alkyl chlorides, and for the abnormally high boiling points of water, alcohols, amines, and
amides. While the forces responsible for these increases in boiling points are all van der Waals
forces, the forces can be further subdivided in accordance to their source and intensity. Secondary
forces include London dispersion forces, induced permanent forces, and dipolar forces, including
hydrogen bonding.
All molecules, including nonpolar molecules such as heptane and polyethylene, are attracted to
each other by weak London or dispersion forces that result from induced dipole- induced dipole
interactions. The temporary or transient dipoles are due to instantaneous fluctuations in the electron
cloud density. The energy range of these forces is fairly constant and about 8 kJ/mol. This force is
independent of temperature and is the major force between chains in largely nonpolar polymers
such as those in classical elastomers and soft plastics such as PE.
9/14/2010 3:35:56 PM
K10478.indb 31 9/14/2010 3:35:56 PM
K10478.indb 31