Page 63 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 63
26 Carraher’s Polymer Chemistry
H
R H H
H
H H
H H H
H H
H H
H H CH
H H H 3
H H H H
H H H H H H H H
H H
H H H H H H
H H H H H H
H H H H
H H H H H
H H H H H H H
H H
H H H H
H
H H H
H H H H
H H H H
H
H H H
H H H H
H H H
H H
H
H R H
H H H C
3
HDPE Decane
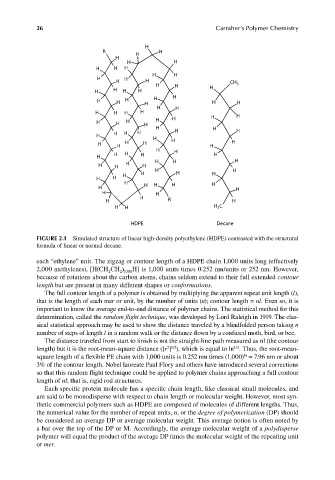
FIGURE 2.1 Simulated structure of linear high-density polyethylene (HDPE) contrasted with the structural
formula of linear or normal decane.
each “ethylene” unit. The zigzag or contour length of a HDPE chain 1,000 units long (effectively
2,000 methylenes), [H(CH CH ) H] is 1,000 units times 0.252 nm/units or 252 nm. However,
2
2 1000
because of rotations about the carbon atoms, chains seldom extend to their full extended contour
length but are present in many different shapes or conformations.
The full contour length of a polymer is obtained by multiplying the apparent repeat unit length (l),
that is the length of each mer or unit, by the number of units (n); contour length = nl. Even so, it is
important to know the average end-to-end distance of polymer chains. The statistical method for this
determination, called the random fl ight technique, was developed by Lord Raleigh in 1919. The clas-
sical statistical approach may be used to show the distance traveled by a blindfolded person taking n
number of steps of length l in a random walk or the distance flown by a confused moth, bird, or bee.
The distance traveled from start to finish is not the straight-line path measured as nl (the contour
1/2
2 1/2
length) but it is the root-mean-square distance ([r ] ), which is equal to ln . Thus, the root-mean-
½
square length of a flexible PE chain with 1,000 units is 0.252 nm times (1,000) = 7.96 nm or about
3% of the contour length. Nobel laureate Paul Flory and others have introduced several corrections
so that this random flight technique could be applied to polymer chains approaching a full contour
length of nl; that is, rigid rod structures.
Each specific protein molecule has a specific chain length, like classical small molecules, and
are said to be monodisperse with respect to chain length or molecular weight. However, most syn-
thetic commercial polymers such as HDPE are composed of molecules of different lengths. Thus,
the numerical value for the number of repeat units, n, or the degree of polymerization (DP) should
be considered an average DP or average molecular weight. This average notion is often noted by
a bar over the top of the DP or M. Accordingly, the average molecular weight of a polydisperse
polymer will equal the product of the average DP times the molecular weight of the repeating unit
or mer.
9/14/2010 3:35:54 PM
K10478.indb 26
K10478.indb 26 9/14/2010 3:35:54 PM