Page 64 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 64
Polymer Structure (Morphology) 27
In organic chemistry, it is customary to call a nonlinear molecule, like isobutane, a branched
compound. However, polymer scientists use the term pendant group to label any group present on
the repeat unit. Thus, polypropylene (PP)
CH 3
(2.5)
( CH CH )
2
has a methyl group as a pendant unit, but PP is designated as a linear polymer. In contrast, low-
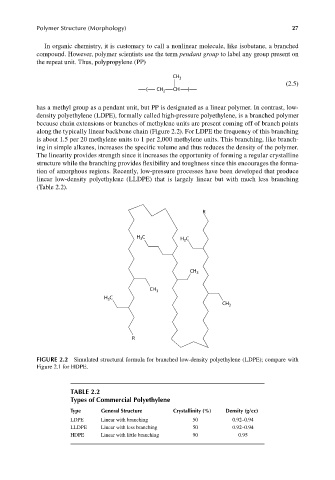
density polyethylene (LDPE), formally called high-pressure polyethylene, is a branched polymer
because chain extensions or branches of methylene units are present coming off of branch points
along the typically linear backbone chain (Figure 2.2). For LDPE the frequency of this branching
is about 1.5 per 20 methylene units to 1 per 2,000 methylene units. This branching, like branch-
ing in simple alkanes, increases the specific volume and thus reduces the density of the polymer.
The linearity provides strength since it increases the opportunity of forming a regular crystalline
structure while the branching provides flexibility and toughness since this encourages the forma-
tion of amorphous regions. Recently, low-pressure processes have been developed that produce
linear low-density polyethylene (LLDPE) that is largely linear but with much less branching
(Table 2.2).
R
H C H C
3
3
CH 3
CH 3
H 3 C
CH 3
R
FIGURE 2.2 Simulated structural formula for branched low-density polyethylene (LDPE); compare with
Figure 2.1 for HDPE.
TABLE 2.2
Types of Commercial Polyethylene
Type General Structure Crystallinity (%) Density (g/cc)
LDPE Linear with branching 50 0.92–0.94
LLDPE Linear with less branching 50 0.92–0.94
HDPE Linear with little branching 90 0.95
9/14/2010 3:35:55 PM
K10478.indb 27 9/14/2010 3:35:55 PM
K10478.indb 27