Page 65 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 65
28 Carraher’s Polymer Chemistry
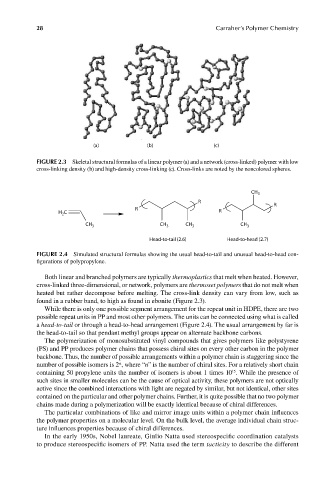
(a) (b) (c)
FIGURE 2.3 Skeletal structural formulas of a linear polymer (a) and a network (cross-linked) polymer with low
cross-linking density (b) and high-density cross-linking (c). Cross-links are noted by the noncolored spheres.
CH 3
R
R
R
H C R
2
CH CH CH
CH 3 3 3 3
Head-to-tail (2.6) Head-to-head (2.7)
FIGURE 2.4 Simulated structural formulas showing the usual head-to-tail and unusual head-to-head con-
figurations of polypropylene.
Both linear and branched polymers are typically thermoplastics that melt when heated. However,
cross-linked three-dimensional, or network, polymers are thermoset polymers that do not melt when
heated but rather decompose before melting. The cross-link density can vary from low, such as
found in a rubber band, to high as found in ebonite (Figure 2.3).
While there is only one possible segment arrangement for the repeat unit in HDPE, there are two
possible repeat units in PP and most other polymers. The units can be connected using what is called
a head-to-tail or through a head-to-head arrangement (Figure 2.4). The usual arrangement by far is
the head-to-tail so that pendant methyl groups appear on alternate backbone carbons.
The polymerization of monosubstituted vinyl compounds that gives polymers like polystyrene
(PS) and PP produces polymer chains that possess chiral sites on every other carbon in the polymer
backbone. Thus, the number of possible arrangements within a polymer chain is staggering since the
n
number of possible isomers is 2 , where “n” is the number of chiral sites. For a relatively short chain
15
containing 50 propylene units the number of isomers is about 1 times 10 . While the presence of
such sites in smaller molecules can be the cause of optical activity, these polymers are not optically
active since the combined interactions with light are negated by similar, but not identical, other sites
contained on the particular and other polymer chains. Further, it is quite possible that no two polymer
chains made during a polymerization will be exactly identical because of chiral differences.
The particular combinations of like and mirror image units within a polymer chain infl uences
the polymer properties on a molecular level. On the bulk level, the average individual chain struc-
ture influences properties because of chiral differences.
In the early 1950s, Nobel laureate, Giulio Natta used stereospecific coordination catalysts
to produce stereospecific isomers of PP. Natta used the term tacticity to describe the different
9/14/2010 3:35:55 PM
K10478.indb 28 9/14/2010 3:35:55 PM
K10478.indb 28