Page 98 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 98
Molecular Weight of Polymers 61
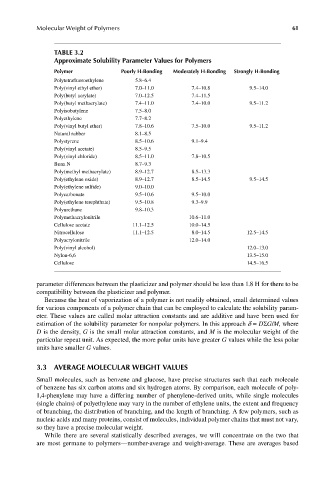
TABLE 3.2
Approximate Solubility Parameter Values for Polymers
Polymer Poorly H-Bonding Moderately H-Bonding Strongly H-Bonding
Polytetrafl uoroethylene 5.8–6.4
Poly(vinyl ethyl ether) 7.0–11.0 7.4–10.8 9.5–14.0
Poly(butyl acrylate) 7.0–12.5 7.4–11.5
Poly(butyl methacrylate) 7.4–11.0 7.4–10.0 9.5–11.2
Polyisobutylene 7.5–8.0
Polyethylene 7.7–8.2
Poly(vinyl butyl ether) 7.8–10.6 7.5–10.0 9.5–11.2
Natural rubber 8.1–8.5
Polystyrene 8.5–10.6 9.1–9.4
Poly(vinyl acetate) 8.5–9.5
Poly(vinyl chloride) 8.5–11.0 7.8–10.5
Buna N 8.7–9.3
Poly(methyl methacrylate) 8.9–12.7 8.5–13.3
Poly(ethylene oxide) 8.9–12.7 8.5–14.5 9.5–14.5
Poly(ethylene sulfi de) 9.0–10.0
Polycarbonate 9.5–10.6 9.5–10.0
Poly(ethylene terephthate) 9.5–10.8 9.3–9.9
Polyurethane 9.8–10.3
Polymethacrylonitrile 10.6–11.0
Cellulose acetate 11.1–12.5 10.0–14.5
Nitrocellulose 11.1–12.5 8.0–14.5 12.5–14.5
Polyacrylonitrile 12.0–14.0
Poly(vinyl alcohol) 12.0–13.0
Nylon-6,6 13.5–15.0
Cellulose 14.5–16.5
parameter differences between the plasticizer and polymer should be less than 1.8 H for there to be
compatibility between the plasticizer and polymer.
Because the heat of vaporization of a polymer is not readily obtained, small determined values
for various components of a polymer chain that can be employed to calculate the solubility param-
eter. These values are called molar attraction constants and are additive and have been used for
estimation of the solubility parameter for nonpolar polymers. In this approach δ = DΣG/M, where
D is the density, G is the small molar attraction constants, and M is the molecular weight of the
particular repeat unit. As expected, the more polar units have greater G values while the less polar
units have smaller G values.
3.3 AVERAGE MOLECULAR WEIGHT VALUES
Small molecules, such as benzene and glucose, have precise structures such that each molecule
of benzene has six carbon atoms and six hydrogen atoms. By comparison, each molecule of poly-
1,4-phenylene may have a differing number of phenylene-derived units, while single molecules
(single chains) of polyethylene may vary in the number of ethylene units, the extent and frequency
of branching, the distribution of branching, and the length of branching. A few polymers, such as
nucleic acids and many proteins, consist of molecules, individual polymer chains that must not vary,
so they have a precise molecular weight.
While there are several statistically described averages, we will concentrate on the two that
are most germane to polymers—number-average and weight-average. These are averages based
9/14/2010 3:36:18 PM
K10478.indb 61 9/14/2010 3:36:18 PM
K10478.indb 61