Page 103 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 103
66 Carraher’s Polymer Chemistry
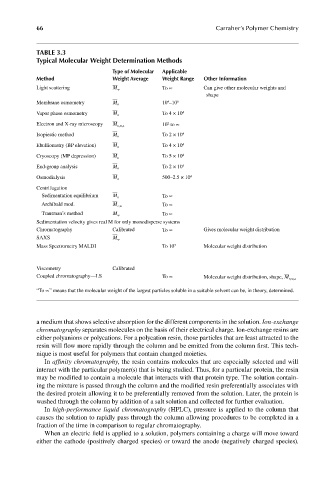
TABLE 3.3
Typical Molecular Weight Determination Methods
Type of Molecular Applicable
Method Weight Average Weight Range Other Information
Light scattering M w To ∝ Can give other molecular weights and
shape
4
Membrane osmometry M n 10 –10 6
Vapor phase osmometry M n To 4 × 10 4
2
Electron and X-ray microscopy M n,w,z 10 to ∝
Isopiestic method M n To 2 × 10 4
Ebulliometry (BP elevation) M n To 4 × 10 4
Cryoscopy (MP depression) M n To 5 × 10 4
End-group analysis M n To 2 × 10 4
Osmodialysis M n 500–2.5 × 10 4
Centrifugation
Sedimentation equilibrium M z To ∝
Archibald mod. M z,w To ∝
Trautman’s method M w To ∝
Sedimentation velocity gives real M for only monodisperse systems
Chromatography Calibrated To ∝ Gives molecular weight distribution
SAXS M w
Mass Spectrometry MALDI To 10 7 Molecular weight distribution
Viscometry Calibrated
Coupled chromatography—LS To ∝ Molecular weight distribution, shape, M n,w,z
“To ∝” means that the molecular weight of the largest particles soluble in a suitable solvent can be, in theory, determined.
a medium that shows selective absorption for the different components in the solution. Ion-exchange
chromatography separates molecules on the basis of their electrical charge. Ion-exchange resins are
either polyanions or polycations. For a polycation resin, those particles that are least attracted to the
resin will flow more rapidly through the column and be emitted from the column first. This tech-
nique is most useful for polymers that contain changed moieties.
In affi nity chromatography, the resin contains molecules that are especially selected and will
interact with the particular polymer(s) that is being studied. Thus, for a particular protein, the resin
may be modified to contain a molecule that interacts with that protein type. The solution contain-
ing the mixture is passed through the column and the modified resin preferentially associates with
the desired protein allowing it to be preferentially removed from the solution. Later, the protein is
washed through the column by addition of a salt solution and collected for further evaluation.
In high-performance liquid chromatography (HPLC), pressure is applied to the column that
causes the solution to rapidly pass through the column allowing procedures to be completed in a
fraction of the time in comparison to regular chromatography.
When an electric field is applied to a solution, polymers containing a charge will move toward
either the cathode (positively charged species) or toward the anode (negatively charged species).
9/14/2010 3:36:34 PM
K10478.indb 66
K10478.indb 66 9/14/2010 3:36:34 PM