Page 109 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 109
unfunctionalized alkenes a, b-unsaturated esters 95
[6]
shows the same enantioselectivity for the epoxidation . The dioxirane is gen-
erated in situ from potassium monoperoxosulfate and the ketone catalyst (I,
Figure 6.4). During the oxygen-atom transfer reaction from the dioxirane to the
E-alkene (II, Figure 6.4), a `spiro' transition state was proposed to give the (E)-
epoxide.
For this method, all glassware needs to be carefully washed to be free of any
trace of metals which catalyse the decomposition of oxone.
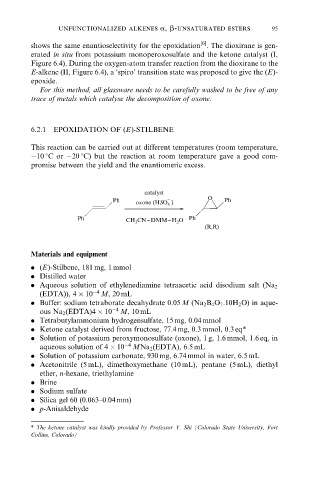
6.2.1 EPOXIDATION OF (E)-STILBENE
This reaction can be carried out at different temperatures (room temperature,
ÿ10 8C or ÿ20 8C) but the reaction at room temperature gave a good com-
promise between the yield and the enantiomeric excess.
catalyst
Ph − O Ph
oxone (HSO )
5
Ph CH 3 CN−DMM−H 2 O Ph
(R,R)
Materials and equipment
. (E)-Stilbene, 181 mg, 1 mmol
. Distilled water
. Aqueous solution of ethylenediamine tetraacetic acid disodium salt (Na 2
(EDTA)), 4 10 ÿ4 M, 20 mL
. Buffer: sodium tetraborate decahydrate 0.05 M (Na 2 B 4 O 7 :10H 2 O) in aque-
ous Na 2 (EDTA)4 10 ÿ4 M, 10 mL
. Tetrabutylammonium hydrogensulfate, 15 mg, 0.04 mmol
. Ketone catalyst derived from fructose, 77.4 mg, 0.3 mmol, 0.3 eq*
. Solution of potassium peroxymonosulfate (oxone), 1 g, 1.6 mmol, 1.6 eq, in
aqueous solution of 4 10 ÿ4 MNa 2 (EDTA), 6.5 mL
. Solution of potassium carbonate, 930 mg, 6.74 mmol in water, 6.5 mL
. Acetonitrile (5 mL), dimethoxymethane (10 mL), pentane (5 mL), diethyl
ether, n-hexane, triethylamine
. Brine
. Sodium sulfate
. Silica gel 60 (0.063±0.04 mm)
. p-Anisaldehyde
* The ketone catalyst was kindly provided by Professor Y. Shi (Colorado State University, Fort
Collins, Colorado)