Page 111 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 111
unfunctionalized alkenes a, b-unsaturated esters 97
6.2.2 CONCLUSION
Epoxidation using a chiral fructose-derived ketone is easy to carry out, as it
occurs in aqueous conditions. The reactions were performed without any modi-
fication of the published procedure. The glassware has to be free of trace metal,
which can decompose the oxone; the use of a plastic spatula is recommended and
the volumes must be measured using glass-graduated cylinders. Table 6.2 gives
different examples of epoxides which can be obtained using the method pre-
scribed.
Shi's method gives good results for disubstituted E-alkenes compared to the
Jacobsen epoxidation previously described, which is more specific for disub-
stituted Z-alkenes. Concerning the epoxidation of trisubstituted alkenes, the
epoxidation of 1-phenyl-1-cyclohexene could not be validated because of
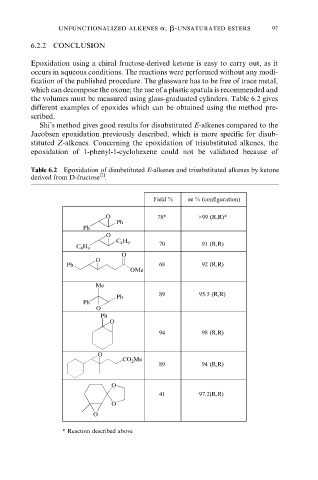
Table 6.2 Epoxidation of disubstituted E-alkenes and trisubstituted alkenes by ketone
[2]
derived from D-fructose .
Yield % ee % (configuration)
O 78* >99 (R,R)*
Ph
Ph
O
C 4 H 9
70 91 (R,R)
C 4 H 9
O
O
Ph 68 92 (R,R)
OMe
Me
89 95.5 (R,R)
Ph
Ph
O
Ph
O
94 98 (R,R)
O
CO 2 Me
89 94 (R,R)
O
41 97.2(R,R)
O
O
* Reaction described above