Page 115 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 115
unfunctionalized alkenes a, b-unsaturated esters 101
VI
1
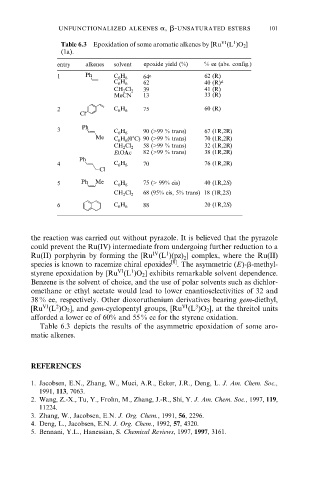
Table 6.3 Epoxidation of some aromatic alkenes by [Ru (L )O 2 ]
(1a).
entry alkenes solvent epoxide yield (%) % ee (abs. config.)
1 Ph C 6 H 6 64 c 62 (R)
62 40 (R) d
C 6 H 6
39 41 (R)
CH 2 Cl 2
MeCN 13 33 (R)
2 C 6 H 6 75 60 (R)
Cl
3 Ph 90 (>99 % trans) 67 (1R,2R)
C 6 H 6
Me C 6 H 6 (08C) 90 (>99 % trans) 70 (1R,2R)
58 (>99 % trans) 32 (1R,2R)
CH 2 Cl 2
EtOAc 82 (>99 % trans) 38 (1R,2R)
Ph
4 C 6 H 6 70 76 (1R,2R)
Cl
5 Ph Me C 6 H 6 75 (> 99% cis) 40 (1R,2S)
68 (95% cis, 5% trans) 18 (1R,2S)
CH 2 Cl 2
6 C 6 H 6 88 20 (1R,2S)
the reaction was carried out without pyrazole. It is believed that the pyrazole
could prevent the Ru(IV) intermediate from undergoing further reduction to a
1
IV
Ru(II) porphyrin by forming the [Ru (L )(pz) ] complex, where the Ru(II)
2
[8]
species is known to racemize chiral epoxides . The asymmetric (E)-b-methyl-
VI
1
styrene epoxidation by [Ru (L )O 2 ] exhibits remarkable solvent dependence.
Benzene is the solvent of choice, and the use of polar solvents such as dichlor-
omethane or ethyl acetate would lead to lower enantioselectivities of 32 and
38 % ee, respectively. Other dioxoruthenium derivatives bearing gem-diethyl,
VI
2
3
VI
[Ru (L )O 2 ], and gem-cyclopentyl groups, [Ru (L )O 2 ], at the threitol units
afforded a lower ee of 60% and 55 % ee for the styrene oxidation.
Table 6.3 depicts the results of the asymmetric epoxidation of some aro-
matic alkenes.
REFERENCES
1. Jacobsen, E.N., Zhang, W., Muci, A.R., Ecker, J.R., Deng, L. J. Am. Chem. Soc.,
1991, 113, 7063.
2. Wang, Z.-X., Tu, Y., Frohn, M., Zhang, J.-R., Shi, Y. J. Am. Chem. Soc., 1997, 119,
11224.
3. Zhang, W., Jacobsen, E.N. J. Org. Chem., 1991, 56, 2296.
4. Deng, L., Jacobsen, E.N. J. Org. Chem., 1992, 57, 4320.
5. Bennani, Y.L., Hanessian, S. Chemical Reviews, 1997, 1997, 3161.