Page 70 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 70
Catalysts for Fine Chemical Synthesis: Hydrolysis, Oxidation and Reduction. Volume 1
Edited by Stan M Roberts and Geraldine Poignant
Copyright 2002 John Wiley & Sons, Ltd.
ISBN: 0-471-98123-0
4 Epoxidation of a, b-Unsaturated
Carbonyl Compounds
CONTENTS
4.1 Non-asymmetric epoxidation . . . . . . . . . . . . . . . . 55
4.2 Asymmetric epoxidation using poly-d-leucine. . . . . . . . . . . 56
4.2.1 Synthesis of leucine N-carboxyanhydride. . . . . . . . . . . . 57
4.2.2 Synthesis of immobilized poly-d-leucine . . . . . . . . . . . . 58
4.2.3 Asymmetric epoxidation of (E)-benzylideneacetophenone. . . . . . . 59
4.2.4 Conclusion . . . . . . . . . . . . . . . . . . . . . 61
4.3 Asymmetric epoxidation using chiral modified diethylzinc. . . . . . . 61
4.3.1 Epoxidation of 2-isobutylidene-1-tetralone . . . . . . . . . . . 62
4.3.2 Conclusion . . . . . . . . . . . . . . . . . . . . . 64
4.4 Asymmetric epoxidation of (E)-benzylideneacetophenone using
the La-(R)-BINOL-Ph 3 PO/cumene hydroperoxide system
K. Daikai, M. Kamaura and J. Inanaga . . . . . . . . . . . . 66
4.4.1 Merits of the system . . . . . . . . . . . . . . . . . . 68
References . . . . . . . . . . . . . . . . . . . . . . . 69
4.1 NON-ASYMMETRIC EPOXIDATION
O O
H O / NaOH O
2
2
R 2 R 1 R 2 R 1
MeOH
rac
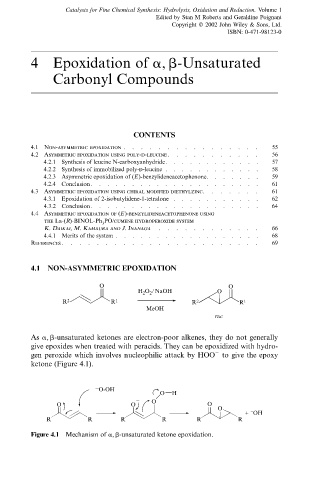
As a, b-unsaturated ketones are electron-poor alkenes, they do not generally
give epoxides when treated with peracids. They can be epoxidized with hydro-
gen peroxide which involves nucleophilic attack by HOO to give the epoxy
ÿ
ketone (Figure 4.1).
− O-OH
O H
− O
O O O
O −
+ OH
R R R R R R
Figure 4.1 Mechanism of a, b-unsaturated ketone epoxidation.