Page 72 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 72
epoxidation of a, b-unsaturated carbonyl compounds 57
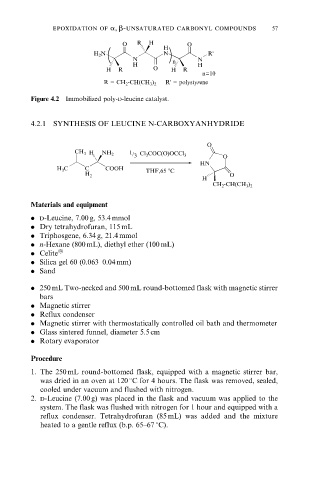
O R H O
H
H N N R'
2
N n N
H H
H R O H R
n=10
R = CH -CH(CH ) R' = polystyrene
3 2
2
Figure 4.2 Immobilized poly-d-leucine catalyst.
4.2.1 SYNTHESIS OF LEUCINE N-CARBOXYANHYDRIDE
O
CH 3 H NH 2 1 / 3 Cl 3 COC(O)OCCl 3
O
HN
H 3 C C COOH
THF,65 8C
H 2 O
H
CH 2 -CH(CH 3 ) 2
Materials and equipment
. d-Leucine, 7.00 g, 53.4 mmol
. Dry tetrahydrofuran, 115 mL
. Triphosgene, 6.34 g, 21.4 mmol
. n-Hexane (800 mL), diethyl ether (100 mL)
. Celite 1
. Silica gel 60 (0.063±0.04 mm)
. Sand
. 250 mL Two-necked and 500 mL round-bottomed flask with magnetic stirrer
bars
. Magnetic stirrer
. Reflux condenser
. Magnetic stirrer with thermostatically controlled oil bath and thermometer
. Glass sintered funnel, diameter 5.5 cm
. Rotary evaporator
Procedure
1. The 250 mL round-bottomed flask, equipped with a magnetic stirrer bar,
was dried in an oven at 120 8C for 4 hours. The flask was removed, sealed,
cooled under vacuum and flushed with nitrogen.
2. d-Leucine (7.00 g) was placed in the flask and vacuum was applied to the
system. The flask was flushed with nitrogen for 1 hour and equipped with a
reflux condenser. Tetrahydrofuran (85 mL) was added and the mixture
heated to a gentle reflux (b.p. 65±67 8C).