Page 76 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 76
epoxidation of a, b-unsaturated carbonyl compounds 61
4.2.4 CONCLUSION
The validation was performed without any modifications. The procedure is
very easy to reproduce and the achieved results correlate with the published
material. The yield is somewhat lower than the published result, though moni-
toring of the reaction by TLC indicated complete consumption of substrate.
This is believed to be due to decreased precipitation during recrystallization.
Because the product is unstable in solution it is recommended that the recrys-
tallization is performed as quickly as possible. Alternatively, the impurities can
be removed by trituration.
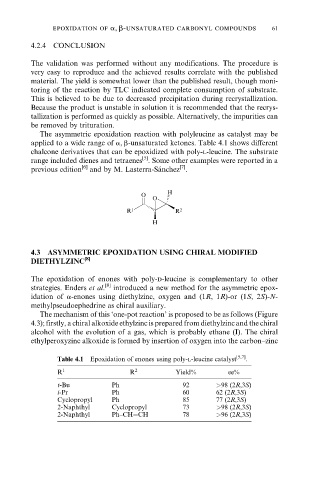
The asymmetric epoxidation reaction with polyleucine as catalyst may be
applied to a wide range of a, b-unsaturated ketones. Table 4.1 shows different
chalcone derivatives that can be epoxidized with poly-l-leucine. The substrate
[5]
range included dienes and tetraenes . Some other examples were reported in a
[7]
previous edition [6] and by M. Lasterra-Sa Ânchez .
H
O
O
R 1 R 2
H
4.3 ASYMMETRIC EPOXIDATION USING CHIRAL MODIFIED
DIETHYLZINC [8]
The epoxidation of enones with poly-d-leucine is complementary to other
strategies. Enders et al. [8] introduced a new method for the asymmetric epox-
idation of a-enones using diethylzinc, oxygen and (1R, 1R)-or (1S, 2S)-N-
methylpseudoephedrine as chiral auxiliary.
The mechanism of this `one-pot reaction' is proposed to be as follows (Figure
4.3); firstly, a chiral alkoxide ethylzinc is prepared from diethylzinc and the chiral
alcohol with the evolution of a gas, which is probably ethane (I). The chiral
ethylperoxyzinc alkoxide is formed by insertion of oxygen into the carbon±zinc
Table 4.1 Epoxidation of enones using poly-l-leucine catalyst [5,7] .
R 1 R 2 Yield% ee%
t-Bu Ph 92 >98 (2R,3S)
i-Pr Ph 60 62 (2R,3S)
Cyclopropyl Ph 85 77 (2R,3S)
2-Naphthyl Cyclopropyl 73 >98 (2R,3S)
2-Naphthyl Ph±CHCH 78 >96 (2R,3S)