Page 77 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 77
62 hydrolysis, oxidation and reduction
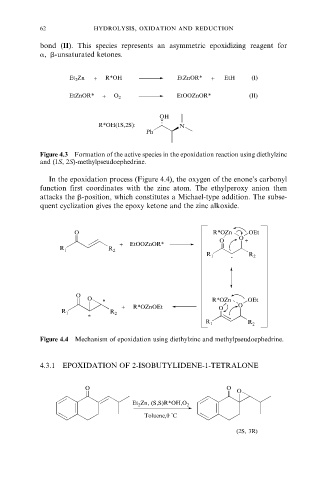
bond (II). This species represents an asymmetric epoxidizing reagent for
a, b-unsaturated ketones.
Et 2 Zn + R*OH EtZnOR* + EtH (I)
EtZnOR* + O 2 EtOOZnOR* (II)
OH
R*OH(1S,2S): N
Ph
Figure 4.3 Formation of the active species in the epoxidation reaction using diethylzinc
and (1S, 2S)-methylpseudoephedrine.
In the epoxidation process (Figure 4.4), the oxygen of the enone's carbonyl
function first coordinates with the zinc atom. The ethylperoxy anion then
attacks the b-position, which constitutes a Michael-type addition. The subse-
quent cyclization gives the epoxy ketone and the zinc alkoxide.
O R*OZn OEt
O
O +
+ EtOOZnOR*
R 1 R 2
-
R 1 R 2
O
O R*OZn OEt
*
+ R*OZnOEt O O
R 1 R 2
*
R 1 R 2
Figure 4.4 Mechanism of epoxidation using diethylzinc and methylpseudoephedrine.
4.3.1 EPOXIDATION OF 2-ISOBUTYLIDENE-1-TETRALONE
O O
O
Et 2 Zn, (S,S)R*OH,O 2
Toluene,0 8 C
(2S, 3R)