Page 81 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 81
66 hydrolysis, oxidation and reduction
4.4 ASYMMETRIC EPOXIDATION OF (E)-
BENZYLIDENEACETOPHENONE USING THE La-(R)-BINOL-Ph 3 PO/
CUMENE HYDROPEROXIDE SYSTEM
K. Daikai, M. Kamaura, and J. Inanaga
Institute for Fundamental Research of Organic Chemistry (IPOC), Kyushu University
Hakozaki, Fukuoka 812±8581, Japan, e-mail: inanaga@ms.ifoc.kyushu-u.ac.jp
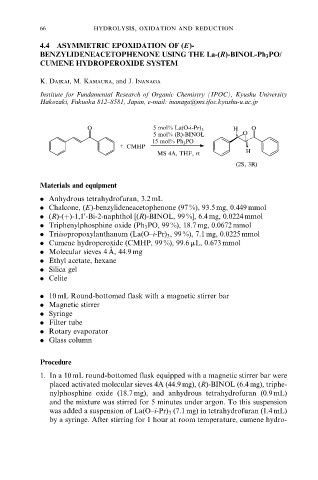
O 5 mol% La(O-i-Pr) 3 H O
5 mol% (R)-BINOL O
15 mol% Ph 3 PO
+ CMHP
MS 4A, THF, rt H
(2S, 3R)
Materials and equipment
. Anhydrous tetrahydrofuran, 3.2 mL
. Chalcone, (E)-benzylideneacetophenone (97 %), 93.5 mg, 0.449 mmol
. (R)-()-1,1 -Bi-2-naphthol [(R)-BINOL, 99 %], 6.4 mg, 0.0224 mmol
0
. Triphenylphosphine oxide (Ph 3 PO, 99 %), 18.7 mg, 0.0672 mmol
. Triisopropoxylanthanum (La(O±i-Pr) 3 , 99 %), 7.1 mg, 0.0225 mmol
. Cumene hydroperoxide (CMHP, 99 %), 99.6 mL, 0.673 mmol
Ê
. Molecular sieves 4 A, 44.9 mg
. Ethyl acetate, hexane
. Silica gel
. Celite
. 10 mL Round-bottomed flask with a magnetic stirrer bar
. Magnetic stirrer
. Syringe
. Filter tube
. Rotary evaporator
. Glass column
Procedure
1. In a 10 mL round-bottomed flask equipped with a magnetic stirrer bar were
placed activated molecular sieves 4A (44.9 mg), (R)-BINOL (6.4 mg), triphe-
nylphosphine oxide (18.7 mg), and anhydrous tetrahydrofuran (0.9 mL)
and the mixture was stirred for 5 minutes under argon. To this suspension
was added a suspension of La(O±i-Pr) 3 (7.1 mg) in tetrahydrofuran (1.4 mL)
by a syringe. After stirring for 1 hour at room temperature, cumene hydro-