Page 85 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 85
Catalysts for Fine Chemical Synthesis: Hydrolysis, Oxidation and Reduction. Volume 1
Edited by Stan M Roberts and Geraldine Poignant
Copyright 2002 John Wiley & Sons, Ltd.
ISBN: 0-471-98123-0
5 Epoxidation of Allylic Alcohols
CONTENTS
5.1 Non-asymmetric epoxidation . . . . . . . . . . . . . . . . 72
5.2 Asymmetric epoxidation using a chiral titanium complex . . . . . . . 73
5.2.1 Epoxidation of cinnamyl alcohol . . . . . . . . . . . . . . 74
5.2.2 Epoxidation of (E)-2-methyl-3-phenyl-2-propenol . . . . . . . . . 76
5.2.3 Epoxidation of (E)-2-hexen-1-ol . . . . . . . . . . . . . . 78
5.2.4 Conclusion . . . . . . . . . . . . . . . . . . . . . 81
5.3 Asymmetric epoxidation of (e)-undec-2-en-1-ol using poly
(octamethylene tartrate)
D.C. Sherrington, J.K. Karjalainen and O.E.O. Hormi . . . . . . . . 81
5.3.1 Synthesis of branched poly(octamethylene-l-()-tartrate) . . . . . . . 81
5.3.2 Asymmetric epoxidation of (E)-undec-2-en-1-ol. . . . . . . . . . 82
References . . . . . . . . . . . . . . . . . . . . . . . 86
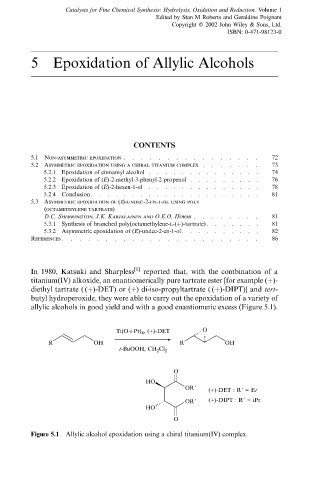
In 1980, Katsuki and Sharpless [1] reported that, with the combination of a
titanium(IV) alkoxide, an enantiomerically pure tartrate ester [for example ()-
diethyl tartrate ( ()-DET) or () di-iso-propyltartrate ( ()-DIPT)] and tert-
butyl hydroperoxide, they were able to carry out the epoxidation of a variety of
allylic alcohols in good yield and with a good enantiomeric excess (Figure 5.1).
Ti(O-i-Pr) 4 , (+)-DET O
R OH R OH
t-BuOOH, CH 2 Cl 2
O
HO
OR9 (+)-DET : R9 = Et
OR9 (+)-DIPT : R9 = iPr
HO
O
Figure 5.1 Allylic alcohol epoxidation using a chiral titanium(IV) complex.