Page 83 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 83
68 hydrolysis, oxidation and reduction
Table 4.4 Epoxidation of a, b-enones using a La-(R)-
BINOL-Ph 3 PO catalyst system [11,12] .
R 1 R 2 ROOH Time/h Yield/% Ee/%
Ph Ph CMHP 0.25 99 99.7
Ph Ph TBHP 0.5 99 96
i-Pr Ph CMHP 4 72 >99.9
i-Pr Ph TBHP 12 67 96
Me Ph CMHP 3 90 99.5
Me Ph TBHP 6 92 93
Ph i-Pr CMHP 3 88 98
Ph i-Pr TBHP 0.5 87 93
Me Ph(CH 2 ) 2 CMHP 18 56 85
Me Ph(CH 2 ) 2 TBHP 1 92 87
The amount of the catalyst can be reduced to 1 mmol% without reducing the
enantioselectivity considerably: 99 % ee (98 % yield) of epoxychalcone was
obtained in the epoxidation of chalcone with CMHP.
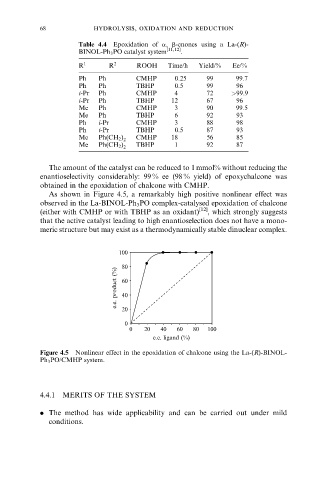
As shown in Figure 4.5, a remarkably high positive nonlinear effect was
observed in the La-BINOL-Ph 3 PO complex-catalysed epoxidation of chalcone
(either with CMHP or with TBHP as an oxidant) [12] , which strongly suggests
that the active catalyst leading to high enantioselection does not have a mono-
meric structure but may exist as a thermodynamically stable dinuclear complex.
100
80
e.e. product (%) 60
40
20
0
0 20 40 60 80 100
e.e. ligand (%)
Figure 4.5 Nonlinear effect in the epoxidation of chalcone using the La-(R)-BINOL-
Ph 3 PO/CMHP system.
4.4.1 MERITS OF THE SYSTEM
. The method has wide applicability and can be carried out under mild
conditions.