Page 80 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 80
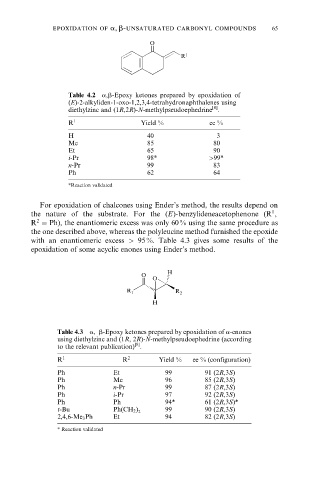
epoxidation of a, b-unsaturated carbonyl compounds 65
O
R 1
Table 4.2 a,b-Epoxy ketones prepared by epoxidation of
(E)-2-alkyliden-1-oxo-1,2,3,4-tetrahydronaphthalenes using
[8]
diethylzinc and (1R,2R)-N-methylpseudoephedrine .
R 1 Yield % ee %
H 40 3
Me 85 80
Et 65 90
i-Pr 98* >99*
n-Pr 99 83
Ph 62 64
*Reaction validated
For epoxidation of chalcones using Ender's method, the results depend on
1
the nature of the substrate. For the (E)-benzylideneacetophenone (R ,
2
R Ph), the enantiomeric excess was only 60 % using the same procedure as
the one described above, whereas the polyleucine method furnished the epoxide
with an enantiomeric excess > 95 %. Table 4.3 gives some results of the
epoxidation of some acyclic enones using Ender's method.
H
O
O
R 1 R 2
H
Table 4.3 a, b-Epoxy ketones prepared by epoxidation of a-enones
using diethylzinc and (1R, 2R)-N-methylpseudoephedrine (according
[8]
to the relevant publication) .
R 1 R 2 Yield % ee % (configuration)
Ph Et 99 91 (2R,3S)
Ph Me 96 85 (2R,3S)
Ph n-Pr 99 87 (2R,3S)
Ph i-Pr 97 92 (2R,3S)
Ph Ph 94* 61 (2R,3S)*
t-Bu Ph(CH 2 ) 99 90 (2R,3S)
2
2,4,6-Me 3 Ph Et 94 82 (2R,3S)
* Reaction validated