Page 90 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 90
76 hydrolysis, oxidation and reduction
In a NMR tube, to a solution of the epoxy alcohol (2.5 mg) in CDCl 3 (0.5 mL)
was added 4-dimethylaminopyridine (5 mg) and (R)-()-a-methoxy-a-(trifluor-
omethyl)phenylacetyl chloride (5 mg). The mixture was allowed to stand over-
night at room temperature. The reaction was monitored by TLC to ensure
1
complete consumption of the starting material. H and 19 F NMR spectra were
carried out on the crude reaction mixture. In the 19 F NMR spectrum, each
enantiomer gave a signal; an additional signal at ÿ71.8 ppm was ascribed to
19
residual MTPA. ( F NMR (250 MHz, CDCl 3 ): d ÿ 70:7 (s, (2R,3R)-enantio-
mer); ÿ72.0 (s, (2S,3S)-enantiomer) ).
1 H NMR (200 MHz, CDCl 3 ): d 7.37±7.27 (m, 5H, Ph); 4.06 (ddd, J 10.5 Hz, J
4.9 Hz, J 3.0 Hz, CH±CH 2 ); 3.94 (d, J 3 Hz, 1H, CH±Ph); 3.81 (dd, J 12.5 Hz,
J 4.9 Hz, 1H, CHaHb); 3.24 (m, 1H, CHaHb), 2.05 (br, 1H, OH).
ÿ1
IR (CHCl 3 , cm ): 3603, 3450 (O±H), 3060 (C±H epoxide), 3010 (C±H
aromatic), 2927, 2875 (C±H aliphatic), 1606, 1461 (CC), 1390, 1308, 1289,
1203 (C±OH, C±O±C), 1103, 1079, 1023, 882, 861, 838.
Mass: calculated for C 9 H 10 O 2 : m/z 150.06808; found [M] 8 150.06781.
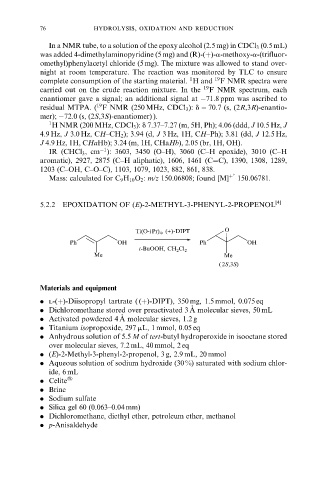
5.2.2 EPOXIDATION OF (E)-2-METHYL-3-PHENYL-2-PROPENOL [4]
Ti(O-iPr) 4 , (+)-DIPT O
Ph OH Ph OH
t-BuOOH, CH 2 Cl 2
Me Me
( 2S,3S)
Materials and equipment
. l-()-Diisopropyl tartrate ( ()-DIPT), 350 mg, 1.5 mmol, 0.075 eq
Ê
. Dichloromethane stored over preactivated 3 A molecular sieves, 50 mL
Ê
. Activated powdered 4 A molecular sieves, 1.2 g
. Titanium isopropoxide, 297 mL, 1 mmol, 0.05 eq
. Anhydrous solution of 5.5 M of tert-butyl hydroperoxide in isooctane stored
over molecular sieves, 7.2 mL, 40 mmol, 2 eq
. (E)-2-Methyl-3-phenyl-2-propenol, 3 g, 2.9 mL, 20 mmol
. Aqueous solution of sodium hydroxide (30 %) saturated with sodium chlor-
ide, 6 mL
. Celite 1
. Brine
. Sodium sulfate
. Silica gel 60 (0.063±0.04 mm)
. Dichloromethane, diethyl ether, petroleum ether, methanol
. p-Anisaldehyde