Page 73 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 73
58 hydrolysis, oxidation and reduction
. The solution remained cloudy due to the insolubility of D-leucine even when
heated in tetrahydrofuran.
3. When the mixture was refluxing, the triphosgene (5.81 g) was added care-
fully, portionwise over 5 minutes. The mixture was heated for 1 hour.
Triphosgene is very toxic. Wear suitable gloves, and eye and face
protection. Handle very carefully in a well-ventilated fume-hood.
The mixture gradually became clearer as the insoluble material was
consumed. If the mixture is not clear after 1 hour continue heating for
another 20 minutes. If the mixture at this stage remained unclear, 0.53 g of
triphosgene was added and heated further.
4. Once the clear mixture had cooled to room temperature, it was then poured
into a 500 mL round-bottomed flask containing n-hexane (400 mL).
1
5. A sintered glass funnel was packed with 2 cm Celite , 1 cm silica gel and
3 cm sand. The n-hexane solution was carefully filtered through this packed
funnel without disturbing the packing material. The filtration was com-
pleted by rinsing the packing with diethyl ether (100 mL).
6. The solvent was removed from the filtrate using a rotary evaporator to
afford a white solid. Dry tetrahydrofuran (30 mL) was added to dissolve
the solid and then n-hexane was added until a white solid precipitated.
Approximately 400 mL of n-hexane was necessary to precipitate the
product.
7. The solution was filtered and the resulting white solid was dried under high
vacuum. This provided leucine N-carboxyanhydride (6.7 g, 80 %); m.p.
76±77 8C, (Lit. [2] 77±79 8C).
This procedure had been scaled up to provide 50 g of the N-carboxyan-
hydride.
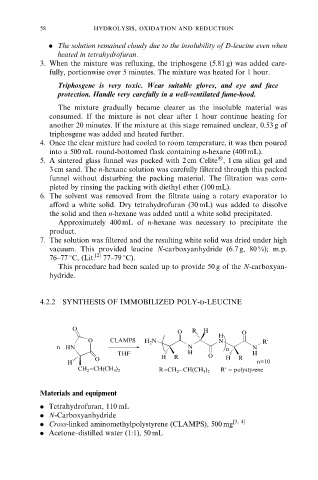
4.2.2 SYNTHESIS OF IMMOBILIZED POLY-d-LEUCINE
O
O R H O
H
O CLAMPS H N N R'
2
n HN N n N
THF H O H
O H R H R
H n=10
−CH(CH )
CH 2 3 2 R=CH −CH(CH ) R' = polystyrene
3 2
2
Materials and equipment
. Tetrahydrofuran, 110 mL
. N-Carboxyanhydride
. Cross-linked aminomethylpolystyrene (CLAMPS), 500 mg [3, 4]
. Acetone±distilled water (1:1), 50 mL